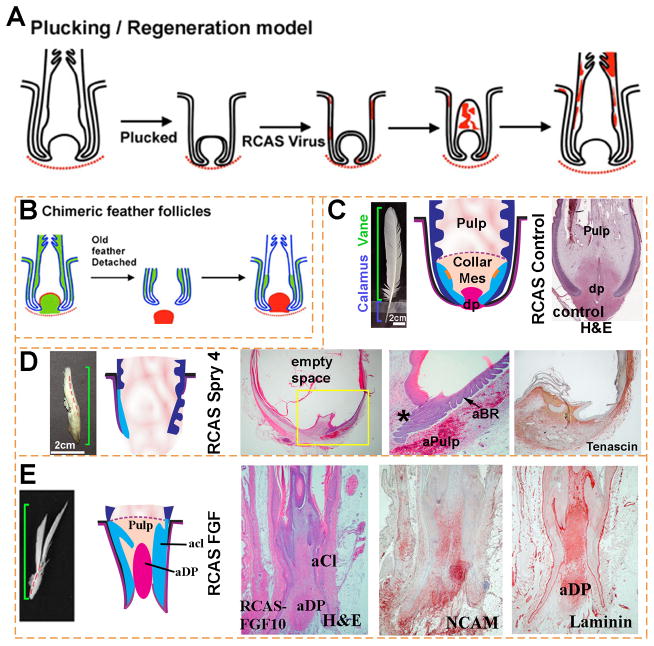
Figure 3. FGF/Sprouty determines the proximal-distal feather morphology and the size of the dermal papilla.
(A, B) Schematic depiction of methods used for studying feather regeneration and morphogenesis. (A). Feathers are plucked to induce the initiation of a new feather. Virus carrying exogenous genes such as β–galactosidase is employed to transduce cells of the newly formed feather. Red color indicates β–galactosidase staining. (B) Classical tissue recombination studies involving microdissection and transplantation of specific components of the follicle. The dermal papilla can be microdissected from the donor follicle and transplanted to the recipient follicle where the dermal papilla has been removed. (C–E) Examples using RCAS sprouty to study the roles of signaling genes on feather morphogenesis. (C) Control feather transduced with RCAS Lac Z. (D) Regenerating feathers transduced with RCAS Spry 4 show miniaturization of the DP, which also becomes Tenascin C negative (compared with Fig. 2A). The perturbed follicle also shows an expanded pulp which is represented by empty space in the section. The follicles also show numerous ectopic branches forming within the follicle and also on the follicle sheath outside of the follicle. (E) Transduction with RCAS FGF10 induces proximal feather structures with a thickened keratinocyte collar and a diffuse dermal papilla which is positive for NCAM and laminin. aCl, abnormal collar; aBr, abnormal barb ridge; aDp, abnormal dermal papilla; aPulp, abnormal pulp.