Abstract
Objective
To examine the association between dehiscence length in patients with superior semicircular canal dehiscence syndrome and their clinical findings, including objective audiometric and vestibular testing results.
Study design
Retrospective study.
Setting
Tertiary referral center.
Patients
Patients included in this study were diagnosed with superior semicircular canal dehiscence syndrome and underwent surgical repair of the dehiscence through middle fossa craniotomy. The dehiscence length was measured intraoperatively in all cases.
Main outcome measures
Correlation between dehiscence length with pure-tone average (PTA), average bone-conduction threshold, maximal air-bone gap, cervical vestibular evoked myogenic potential (cVEMP) thresholds, and presenting signs and symptoms.
Results
The correlation between dehiscence length and maximal air-bone gap was statistically significant on both univariate and multivariate regression analyses. The correlations between dehiscence length and PTA, average bone-conduction threshold, cVEMP threshold, and presenting signs and symptoms, were not statistically significant.
Conclusions
The dehiscence length correlated positively with the maximal air-bone gap in patients with superior semicircular canal dehiscence. The correlation was statistically significant. The dehiscence length did not correlate with the other variables examined in this study.
Introduction
Superior semicircular canal dehiscence (SCD) syndrome is a recently described condition in which patients present with vertigo and oscillopsia (1–4). The etiology of this syndrome was localized to a dehiscence of the bone covering the superior semicircular canal. The abnormal communication between the superior semicircular canal and the brain can result in vertigo and oscillopsia induced by loud sounds (Tullio phenomenon), by changes in pressure in the ear canal that are transmitted to the middle ear (Hennebert sign), or by Valsalva maneuvers (1,2,4). Some patients with SCD also present with auditory manifestations. Bone conduction thresholds on audiometry can be less than 0 dB normal hearing level, and the air conduction thresholds are frequently elevated, resulting in an air–bone gap, particularly at low frequencies (5,6). In fact, patients with SCD can present primarily with auditory symptoms, without any vestibular complaints. The reason as to why some patients present predominantly with auditory complaints, and some present predominantly with vestibular complaints, while others present with a mixture of both, remains unclear. A recent study found that patients with longer dehiscence length (≥2.5 mm) measured radiographically presented predominantly with both vestibular and auditory abnormalities, whereas patients with smaller dehiscence length present with either auditory or vestibular dysfunction (7). Another study also showed a positive correlation between radiographic dehiscence length and size of the air-bone gap in SCD patients (8). In this study, we examine the association between dehiscence length and clinical findings in SCD patients. In contrast to previous studies, dehiscence size is measured directly at the time of surgery in this study.
Methods
The data used in this study were obtained from a database of cases with vestibular, audiometric, and imaging data in patients with inner ear disorders, including superior canal dehiscence syndrome. It qualified for exemption from an institutional review board protocol based on United States Department of Health and Human Services criteria 45 CFR 46.101(b4). There were 225 patients with superior canal dehiscence syndrome in this database (from 03/1996 to 12/2010), 117 of whom have had surgery for SCD repair. Out of these patients, 106 patients had intraoperative measurement of dehiscence length. Twenty patients were excluded from the study due to prior otologic surgery (stapedectomy, perilymphatic fistula repair, prior SCD repair). These patients were excluded because the audiometric or vestibular findings could be confounded by the previous surgery and, therefore, might not be solely due to the dehiscence. One patient was excluded due to a history of sudden profound sensorineural hearing loss in the same ear as SCD prior to presenting to our clinic for evaluation. The remaining 85 patients were included in this study. The average age was 46 years (range 29–66). Forty-three were men and 42 were women. Forty-one dehiscences involved the right superior semicircular canal, and 44 involved the left.
The diagnosis of SCD was based on clinical testing and physical examination findings. Clinical findings were retrieved from the database. Symptoms considered included chronic disequilibrium, autophony, sound- and pressure-induced vertigo, pulsatile tinnitus, and positional vertigo. Clinical signs evaluated included nystagmus induced by tones with an audiometer, Valsalva maneuvers, or external auditory canal pressure. Head tilt induced by tones with an audiometer was also examined. These 10 signs and symptoms were evaluated, and the total number of positive findings in each patient was recorded (from a minimum of zero to a maximum of 10 signs and symptoms). Extended bone-conduction audiometry (using an audiometer specially calibrated to detect bone conduction thresholds as low as −20 dB nHL) was used to measure conductive hyperacusis. All patients had audiograms prior to surgery. Preoperative cervical vestibular evoked myogenic potential (cVEMP) responses were measured in 73 patients. Computed tomography of the temporal bones with reconstructions in the plane of the superior semicircular canal as well as orthogonal to that plane was performed in every patient. In all cases, the CT scans were consistent with SCD on the side for surgery.
All patients in this study underwent SCD repair via a middle fossa craniotomy as previously described (9). The location of the SCD in the middle fossa was found with the aid of image navigation. The size of the dehiscence was measured using a small scale several millimeters long laid next to the dehiscence and viewed under high magnification. The length of the dehiscence was measured under the operating microscope with an estimated error of 0.2 mm.
The dehiscence was plugged by packing small pieces of previously harvested temporal is fascia into the lumen of the bony superior canal on both ends of the dehiscence. Bone chips and bone pate were used to reinforce the plugs. After surgery, patients remained under observation in a neurosurgical intensive care unit overnight. Patients were discharged from the hospital on the 2nd or 3rd postoperative day. Dexamethasone 6–8 mg was given 3–4 times daily for the first 24 hours. If there was no evidence of sensorineural hearing loss or pan-labyrinthine hypofunction on bedside testing, then the steroids were tapered over 5 days. Otherwise, the taper was prolonged for 10–14 days. Bedside testing consisted of the Weber and Rinne tuning fork tests and assessment of the vestibuloocular reflexes with rapid rotary head impulses (head thrusts).
Statistical analyses for this study were performed using STATA (StataCorp LP, College Station, TX) and Excel (Microsoft, Seattle, WA). Simple linear and Poisson regression analyses were used to examine the association of dehiscence length and clinical findings, including audiometric and vestibular test results. Statistical significance was defined as p < .05. Sample size calculation showed that for α =0.05 and β =0.8, 85 patients were needed to detect a correlation coefficient of 0.3.
Results
1. Pure-tone average
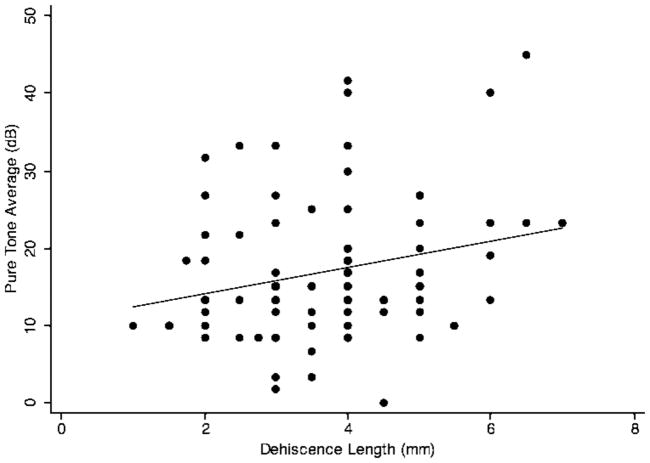
Patients with SCD often present with hearing loss. Pure-tone average (PTA) is a commonly used measure of the degree of hearing loss in patients. It is calculated by taking the average of air-conduction thresholds at 500, 1000 and 2000 Hz. Figure 1 shows the scatter plot of PTA vs. dehiscence length. The linear regression line showed a positive association between PTA and dehiscence length. Simple linear regression analysis showed the Pearson’s correlation coefficient (r) to be 0.16 (p=.15). Since age and sex are both important predictors of hearing loss (10), it is important to control for them when examining the association between PTA and dehiscence length. Multivariate regression analysis of PTA and dehiscence length, controlling for age and sex (Table 1), showed the regression coefficient (β1) to be 1.36 (p=.08). Coefficient of determination (R2) for the multivariate model was 0.15, meaning that only 15% of the variance in PTA could be accounted for by the combination of age, sex, and dehiscence length.
FIG. 1.
Simple linear regression of SCD length and pure-tone average (PTA). The linear regression line shows a positive correlation between SCD length and PTA (slope=1.17). The Pearson’s correlation coefficient is not statistically significant (p = .15).
Table 1.
| Mean | Range | SD | SLR | MLR | R2 | |
|---|---|---|---|---|---|---|
| SCD length (mm) | 3.64 | 1 to 7 | 1.29 | |||
| PTA (dB HL) | 17.6 | 0 to 45 | 9.52 | 0.15 | 0.08 | 0.15 |
| Ave BC (dB HL) | 1.00 | −10 to 35 | 7.83 | 0.67 | 0.63 | 0.11 |
| Max ABG (dB HL) | 26.7 | 0 to 55 | 12.7 | 0.046 | 0.02 | 0.11 |
| cVEMP (dB) | 73.3 | 45 to 106 | 10.2 | 0.26 | 0.23 | 0.025 |
Abbreviations: PTA=Pure-tone average; Ave BC=Average bone-conduction threshold; Max ABG=Maximal air-bone gap; cVEMP=cVEMP threshold; SD=Standard deviation; SLR=Simple linear regression coefficient p-value; MLR=Multivariate linear regression coefficient p-value; R2=Coefficient of determination for the multivariate model.
2. Bone conduction thresholds
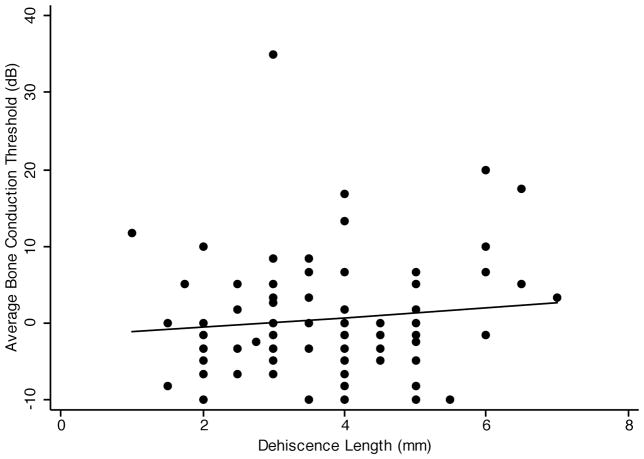
Hyperacusis for bone-conducted sounds is often seen in SCD. This is indicated by bone conduction thresholds below 0 dB nHL. We examined the correlation between the average low-frequency bone conduction threshold (the average of bone conduction thresholds at 250 Hz, 500 Hz, and 1000 Hz) and the dehiscence length. Figure 2 shows the scatter plot of average bone-conduction threshold vs. dehiscence length. The linear regression line showed a slightly positive association between average bone conduction threshold and dehiscence length. Simple linear regression analysis showed the Pearson’s correlation coefficient to be 0.05 (p=.67). Multivariate regression analysis of average bone conduction threshold and dehiscence length, controlling for age and sex (Table 1), showed the regression coefficient to be 0.31 (p=.63). The R2 for the multivariate model was 0.11. Thus, there was no significant correlation of average bone conduction threshold with dehiscence length.
FIG. 2.
Simple linear regression of SCD length and average bone-conduction thresholds. The linear regression line shows a slightly positive correlation between SCD length and average bone-conduction thresholds (slope = 0.29). The Pearson’s correlation coefficient is not statistically significant (p = .67).
3. Maximal air-bone gap
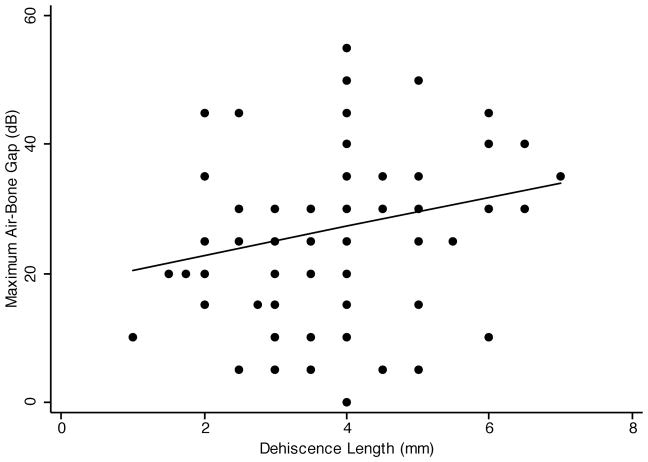
The combination of bone conduction hyperacusis and elevation in air-conduction thresholds results in an air-bone gap. We examined the correlation between maximum air-bone gap and the dehiscence length. Figure 3 shows the scatter plot of maximal air-bone gap vs. dehiscence length. The linear regression line shows a positive association between maximal air-bone gap and dehiscence length. Simple linear regression analysis showed the Pearson’s correlation coefficient to be 0.22 (p=.046). Multivariate regression analysis of maximal air-bone gap and dehiscence length, controlling for age and sex (Table 1), showed the regression coefficient to be 2.45, p=.021). The R2 for the multivariate model was 0.11. Thus, there was a significant correlation between maximal air-bone gap and dehiscence length, but only 11% of the variance in air-bone gap data can be accounted for by the combination of age, sex, and dehiscence length.
FIG. 3.
Simple linear regression of SCD length and maximal air-bone gap. The linear regression line shows a positive correlation between SCD length and maximal air-bone gap (slope = 2.14). The Pearson’s correlation coefficient is statistically significant (p = .046).
4. Vestibular evoked myogenic potentials
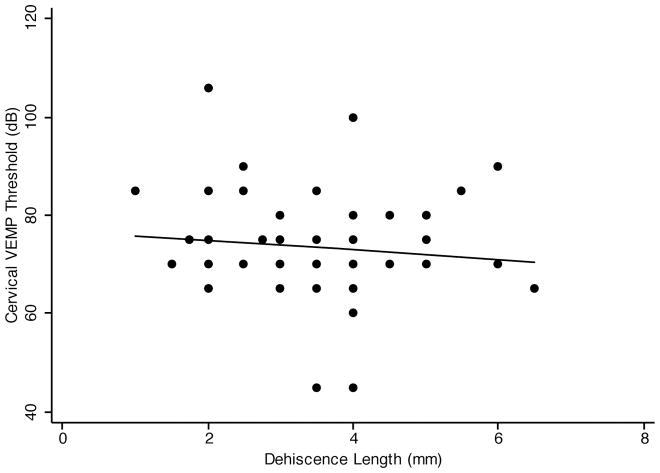
Patients with SCD often have a decreased cervical VEMP threshold. Cervical VEMP threshold was documented in 73 out of 85 patients in our study. Figure 4 shows the scatter plot of cVEMP threshold vs. dehiscence length. The linear regression line shows a negative association between cVEMP threshold and dehiscence length. Simple linear regression analysis showed that cVEMP threshold was not significantly associated with the dehiscence length (Pearson’s correlation coefficient −0.13, p=.26). Since age and sex are both predictors of VEMP threshold (11,12), it is important to control for them when examining the association between cVEMP threshold and dehiscence length. Multivariate regression analysis of cVEMP threshold and dehiscence length, controlling for age and sex (Table 1), showed the regression coefficient to be −1.22 (p=.23). Coefficient of determination (R2) for the multivariate model was 0.025. Thus, although cVEMP thresholds may be reduced in SCD syndrome compared to controls, there was no significant correlation between cVEMP threshold and dehiscence length.
FIG. 4.
Simple linear regression of SCD length and cVEMP threshold. The linear regression line shows a negative correlation between SCD length and cVEMP threshold (slope = −1.11). The Pearson’s correlation coefficient is not statistically significant (p = .26).
5. SCD signs and symptoms
We examined whether dehiscence length was associated with the total number of vestibular and auditory signs and symptoms in SCD patients. Each SCD patients in this study was assessed for the presence of 7 vestibular and 3 auditory signs and symptoms (listed in the methods section). The median number of signs and symptoms in our patient cohort was 6 (range 1–10). One patient had only vestibular complaints, while 2 had only auditory complaints. The remaining 82 patients experienced both auditory and vestibular signs and symptoms. Poisson regression, a correlation technique used for count data, was used to analyze the association between the number of presenting signs and symptoms and dehiscence length. The Poisson regression coefficient was 0.03 and was not statistically significant (p=.39).
Discussion
Superior canal dehiscence syndrome was first described by Minor et al. in 1998 after examining a group of patients presenting with vertigo and oscillopsia (1). High resolution temporal bone CT scan of these patients confirmed a dehiscence of bone covering the superior semicircular canal. It was later found that some of these patients can also present predominantly with auditory signs and symptoms, especially low-frequency conductive hearing loss and hyperacusis to bone-conducted sounds (5,6). The mechanism in which SCD causes its clinical manifestations can be explained by the third window hypothesis. Normally, the inner ear has two mobile windows: the oval and the round windows. In the presence of SCD, a third mobile window is created in the inner ear, which acts as a shunt pathway for sound energy to travel through the labyrinth instead of the cochlea. This can lead to both auditory and vestibular signs and symptoms (1,2,13,14).
The reason why SCD causes predominantly vestibular findings in some patients, auditory complaints in others, and a combination of both vestibular and auditory abnormalities in yet another group of patients, is unclear. Some have postulated that dehiscence size and/or location may determine the clinical presentation in SCD. It may seem logical to assume that larger dehiscences would cause an increase in the number and severity of auditory and vestibular signs and symptoms, since the shunt pathway created by a larger dehiscence might be expected to cause more significant effects on inner ear mechanics. However, a mechano-acoustic model analysis of inner ear mechanics suggests otherwise.
Songer and Rosowski (15) developed a mechano-acoustic model of SCD to examine the effect of SCD length on hearing. In this model, a two-port network was used, and the transmission matrix parameters were derived using measurements in chinchilla ears. A lumped-element model of SCD was created based on anatomical parameters calculated from a histological reconstruction of the chinchilla inner ear. According to this model, when the dehiscence is very small, there should be very little effect on auditory sensitivity (as defined by cochlear potential normalized by sound pressure). As the dehiscence size approaches the cross-sectional area of the superior canal lumen, the auditory sensitivity decreases. However, once the dehiscence size exceeds the cross-sectional area of the superior canal lumen, there is little further effect on auditory sensitivity.
In this study, we examined the association between dehiscence length and clinical manifestations in SCD patients. Our analysis showed that only maximal air-bone gap was statistically significantly associated with dehiscence length. All other variables that we examined, including PTA, average bone-conduction threshold, cVEMP threshold, and the total number of auditory and vestibular signs and symptoms, did not correlate significantly with SCD length. Since this study only focused on patients who underwent surgical repair of SCD, and since only those who were affected more severely would seek surgical treatment for SCD, it is possible that the dehiscence length of our study cohort may be too large to show any effect of dehiscence length on most of the studied variables. Specifically, even though the smallest dehiscence length we measured was approximately 1 mm, most of the dehiscences were longer than 2 mm in our study cohort. Igarashi (16) found that the diameter of the human semicircular canal was 1.44 mm. Thus, the dehiscence lengths in this cohort of patients who underwent surgery may have largely exceeded a value below which graded effects on auditory or vestibular function might be seen. However, the fact that there was a statistically significant correlation between maximal air-bone gap and dehiscence length suggests that even though the effect of dehiscence length on air conduction and bone conduction thresholds individually may be small, when the dehiscence was large, their combined effects are still be detectable.
A recent study by Pframmatter et al. (7) examined the correlation between dehiscence length and patient symptoms in 27 patients with SCD. The dehiscence length was estimated using temporal bone CT scan alone in their study. They found that patients with larger dehiscences (≥2.5 mm) presented predominantly with both vestibular and auditory abnormalities, whereas patients with smaller dehiscences (<2.5 mm) presented with either auditory or vestibular dysfunction. They also found that patients with SCD length ≥2.5 mm were more likely to have a cVEMP threshold of ≤80 dB, whereas those with SCD length <2.5 mm were more likely to have a cVEMP threshold of >80 dB. In another study (8), Yuen et al. found that the length of dehiscence correlated positively with the size of average air-bone gap (average of air-bone gaps at 500, 1000, and 2000 Hz) in 23 patients with SCD (28 ears), with an R2 of 0.828 when the regression model was fitted quadratically (p<0.001), and R2 of 0.780 when the regression model was fitted linearly (p<0.001). The dehiscence length was also estimated only using temporal bone CT scan.
Our study did not show a significant correlation between the number of auditory and vestibular signs and symptoms with dehiscence length. Since our study only focused on patients who underwent surgical repair of SCD, our patients were likely to be more symptomatic compared to the cohort in the Pframmatter study (7). In fact, 82 out of 85 of the patients in our study (96%) had both auditory and vestibular signs and symptoms, whereas only 78% of the patients showed both auditory and vestibular signs and symptoms in the Pframmatter study. Similarly, it is possible that the mean dehiscence length in the Pframmatter study was shorter than that in our study. Thus, they were able to detect a significant association between dehiscence length and clinical manifestations of SCD. Unfortunately the dehiscence lengths were not stated specifically in that study for comparison. Another possible source of disparity is the different statistical techniques used for data analysis (Poisson regression in our case vs. t-tests in the Pframmatter study).
Our study found a statistically significant association between maximal air-bone gap and dehiscence length. This finding is similar to the study by Yuen et el. (8), though average air-bone gap was used in their study instead. It is important to point out that our data were much more scattered than the Yuen study, as evidenced by the small R2 in our multivariate regression analysis (0.11) compared to theirs (0.78). The R2 of our regression model also did not improve when fitted quadratically (data not shown). One possible source of difference was that patients who had previous SCD repair were included in the Yuen study. In fact, in one case, the patient was included twice (both preoperatively and postoperatively). Since prior otologic surgery is a known risk factor for postoperative hearing (17), inclusion of patients with previous otologic surgery may lead to biased results.
In considering studies correlating dimensions of SCD with signs and symptoms, caution must be exercised when interpreting data obtained from CT measurements but not confirmed at the time of surgery. It is our experience that high-resolution CT commonly overestimates the size of the dehiscence that is actually found at the time of surgery. In the present study, using measurements actually obtained at surgery, we found that the only significant correlation of SCD size with auditory and vestibular signs and symptoms was for the maximal air-bone gap.
One aspect of vestibular function was not investigated in this study: the correlation between dehiscence length and the rotational sensitivity of the dehiscent superior canal. Cremer et al. (3) showed that when dehiscence length exceeded 5 mm, there was a significantly lower gain of the affected canal’s angular vestibuloocular reflex (AVOR) compared to that of normal canals or canals with SCD lengths shorter than 5 mm. This effect has been attributed to “autoplugging” of the canal, i.e., the presumed herniation of dura into the lumen of the dehiscent canal to a sufficient extent to compress the membranous duct and dampen endolymph flow under normal circumstances even before surgery. Demonstrating quantitative AVOR gains requires magnetic search coil or high-speed video recordings that are beyond the scope of this study of a large cohort of patients. The present study does not negate the “autoplugging” effect of large dehiscences on AVOR gain, but it points out that effects of dehiscence length on auditory and vestibular signs and symptoms are otherwise so small or variable that they cannot be detected in a cohort of this size or using these common clinical measures.
Conclusion
In this study, we examined the correlation between SCD length and objective auditory and vestibular measurements in SCD patients. We also examined the correlation between SCD length and the presenting signs and symptoms in these patients. We found that there was a statistically significant correlation between SCD length and maximal air-bone gap. There was no statistically significant correlation between SCD length and PTA, average bone-conduction thresholds, cVEMP thresholds, and the total number of auditory and vestibular signs and symptoms.
Footnotes
Disclosures: All authors have no conflict of interest to disclose
References
- 1.Minor LB, Solomon D, Zinreich JS, et al. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–58. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Minor LB. Superior canal dehiscence syndrome. Am J Otol. 2000;21:9–19. [PubMed] [Google Scholar]
- 3.Cremer PD, Minor LB, Carey JP, et al. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55:1833–41. doi: 10.1212/wnl.55.12.1833. [DOI] [PubMed] [Google Scholar]
- 4.Minor LB, Cremer PD, Carey JP, et al. Symptoms and signs in superior canal dehiscence syndrome. Ann N Y Acad Sci. 2001;942:259–73. doi: 10.1111/j.1749-6632.2001.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 5.Minor LB, Carey JP, Cremer PD, et al. Dehiscence of bone overlying the superior canal as a cause of apparent conductive hearing loss. Otol Neurotol. 2003;24:270–8. doi: 10.1097/00129492-200303000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Mikulec AA, McKenna MJ, Ramsey MJ, et al. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004;25:121–9. doi: 10.1097/00129492-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Pfammatter A, Darrouzet V, Gartner M, et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol. 2010;31:447–54. doi: 10.1097/MAO.0b013e3181d27740. [DOI] [PubMed] [Google Scholar]
- 8.Yuen HW, Boeddinghaus R, Eikelboom RH, et al. The relationship between the air-bone gap and the size of superior semicircular canal dehiscence. Otolaryngol Head Neck Surg. 2009;141:689–94. doi: 10.1016/j.otohns.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–27. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30:139–45. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- 11.Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001;112:1971–9. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee SK, Cha CI, Jung TS, et al. Age-related differences in parameters of vestibular evoked myogenic potentials. Acta Otolaryngol. 2008;128:66–72. doi: 10.1080/00016480701387108. [DOI] [PubMed] [Google Scholar]
- 13.Chien W, Ravicz ME, Rosowski JJ, et al. Measurements of human middle- and inner-ear mechanics with dehiscence of the superior semicircular canal. Otol Neurotol. 2007;28:250–7. doi: 10.1097/01.mao.0000244370.47320.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosowski JJ, Songer JE, Nakajima HH, et al. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25:323–32. doi: 10.1097/00129492-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Songer JE, Rosowski JJ. A mechano-acoustic model of the effect of superior canal dehiscence on hearing in chinchilla. J Acoust Soc Am. 2007;122:943–51. doi: 10.1121/1.2747158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igarashi M. Dimensional study of the vestibular apparatus. Laryngoscope. 1967;77:1806–1817. doi: 10.1288/00005537-196710000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Limb CJ, Carey JP, Srireddy S, et al. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27:969–80. doi: 10.1097/01.mao.0000235376.70492.8e. [DOI] [PubMed] [Google Scholar]