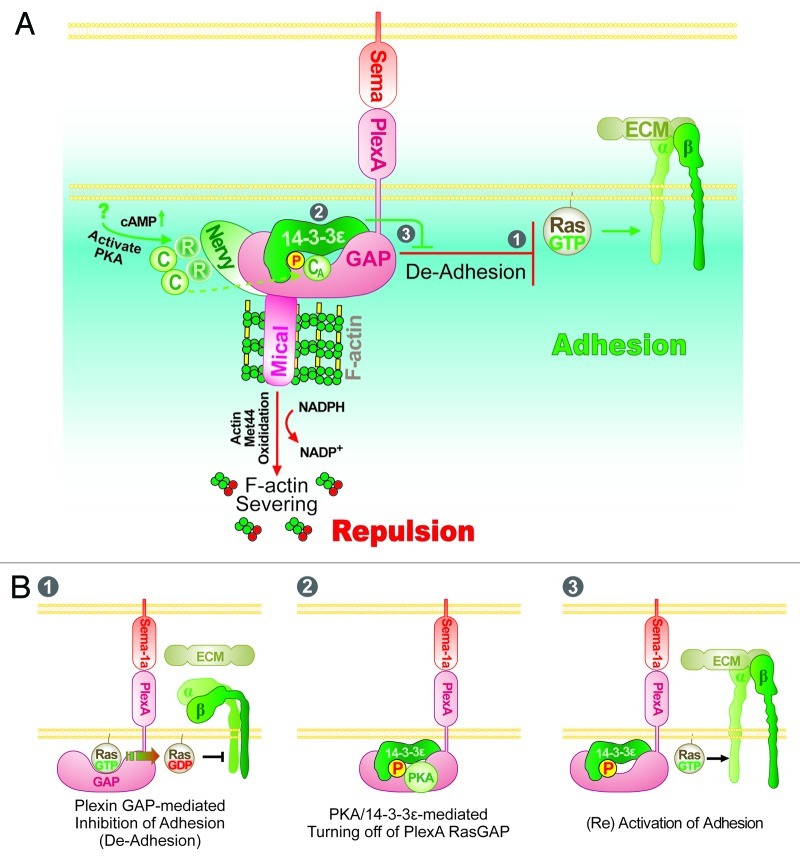
Figure 1. Working model of the mechanisms underlying Drosophila Sema-1a/PlexA-mediated repulsive axon guidance. (A) Drosophila Sema-1a exerts repulsive guidance effects by activating PlexA, which then induces repulsion. In particular, the cytoplasmic region of PlexA interacts with Mical, a novel F-actin disassembly factor that directly oxidizes the Met44 residue of actin to disassemble F-actin and limit actin polymerization. Plexin family proteins also have a GAP domain within their cytoplasmic region, which inactivates members of the Ras/Rap family of small GTPases to inhibit Integrin-mediated cell adhesion (De-Adhesion). Our recent results indicate that this Sema/Plexin “De-adhesion” can be turned-off by phosphorylation of the GAP domain of PlexA by protein kinase A (PKA), which allows binding of 14-3-3ε to PlexA and disrupts the interaction of the PlexA GAP domain with its Ras substrate. These interactions effectively allow Integrin-mediated adhesion to be turned back on (see also B). Several other molecules including Off-track, Gyc76C and Plexin B have also been implicated in this signaling pathway but their mechanistic role in this pathway is not yet clear.51 The circled numbers (1, 2 and 3) refer to the panels in (B) which provide more detail. (B) Panel 1: Plexin RasGAP facilitates GTPase activity of its substrate Ras/Rap family GTPases. Subsequently, GDP-bound inactive Ras is unable to activate Integrin-mediated cell adhesion. Panel 2: PKA phosphorylates a serine residue in the Plexin RasGAP domain and generates a binding site for 14-3-3ε. Panel 3: The interaction between 14-3-3ε and Plexin inhibits Plexin GAP activity by preventing it from associating with its Ras substrate. GTP-bound active Ras is able to activate Integrin-mediated adhesion.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.