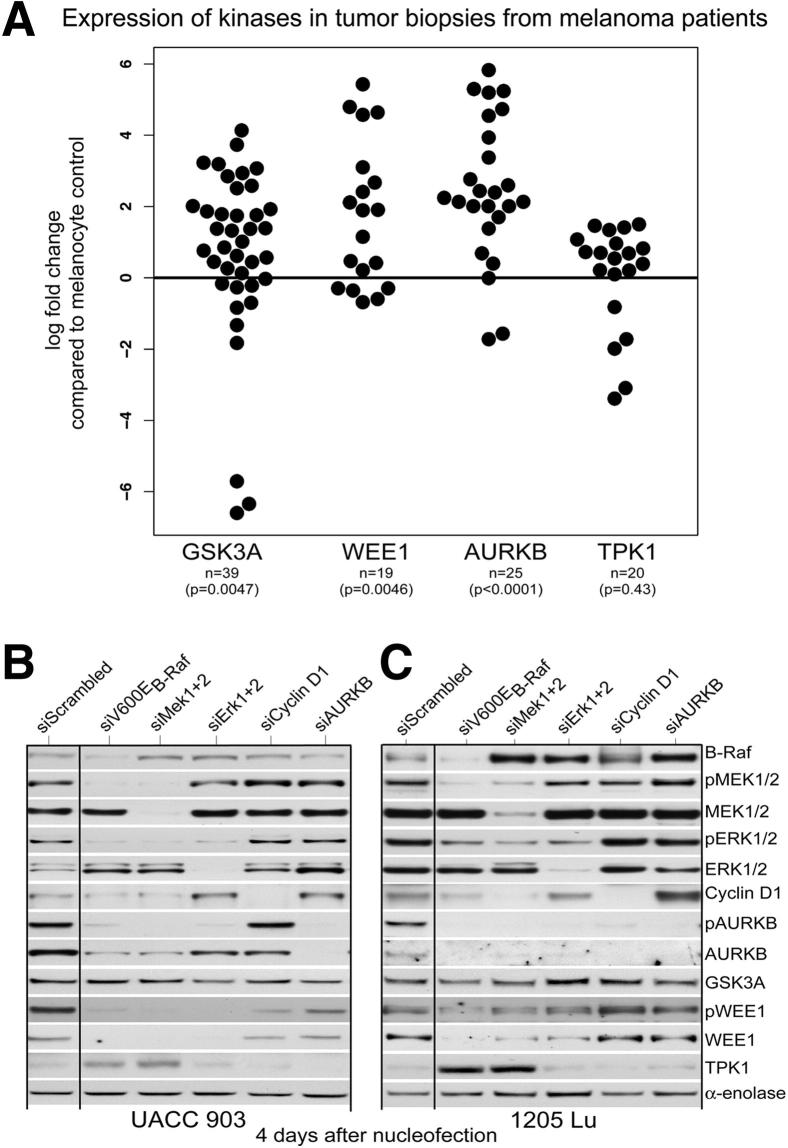
Figure 2.
Expression and demonstration that AURKB and WEE1, but not GSK3A or TPK1, lie downstream of V600EB-RAF in the MAP kinase–signaling pathway. A: Expression of AURKB, WEE1, GSK3A, and TPK1 in tumor biopsy specimens from patients with melanoma. Protein isolated from tumors of patients with melanoma (n = 39) was analyzed for AURKB, WEE1, GSK3A, and TPK1 expression by using Western blot analysis. Results were normalized to α-enolase for equal protein loading and compared with normal human melanocyte controls. Data represent fold changes, relative to melanocytes (melanocytes were normalized to a fold change of one or a log fold change of zero), and were analyzed and graphed on the log scale. Number of samples for AURKB (n = 25, P < 0.0001), WEE1 (n = 19, P = 0.0046), GSK3A (n = 39, P = 0.0047), and TPK1 (n = 20, P = 0.43), two-sided one-sample Wilcoxon signed-rank test. B and C: Decreasing levels of protein expression for each member of the MAP kinase pathway inhibit AURKB or WEE1 protein or activity levels. siRNA was introduced into UACC 903 or 1205 Lu cells to reduce protein levels of V600EB-Raf, MEK1/2, ERK1/2, cyclin D1, and AURKB. The effect on AURKB, pAURKB, WEE1, GSK3A, or TPK1 protein levels was measured. Compared with cells treated with scrambled siRNA, decreasing protein levels of each member of the MAP kinase pathway led to a decrease in AURKB and WEE1 levels. No changes were observed in GSK3A or TPK1 levels. α-Enolase served as a control for equal protein loading. Western blot was reproduced at least two to three times (B).