Abstract
Pseudoxanthoma elasticum (PXE) is a multisystem ectopic mineralization disorder caused by mutations in the ABCC6 gene. Warfarin, a commonly used anticoagulant, is associated with increased mineralization of the arterial blood vessels and cardiac valves. We hypothesized that warfarin may accelerate ectopic tissue mineralization in PXE, with clinical consequences. To test this hypothesis, we developed a model in which Abcc6−/− mice, which recapitulate features of PXE, were fed a diet supplemented with warfarin and vitamin K1. Warfarin action was confirmed by significantly increased serum levels of oxidized vitamin K. For mice placed on a warfarin-containing diet, quantitative chemical and morphometric analyses revealed massive accumulation of mineral deposits in a number of tissues. Mice fed a warfarin-containing diet were also shown to have abundant uncarboxylated form of matrix Gla protein, which allowed progressive tissue mineralization to ensue. To explore the clinical relevance of these findings, 1747 patients with PXE from the approximately 4000 patients in the PXE International database were surveyed about the use of warfarin. Of the 539 respondents, 2.6% reported past or present use of warfarin. Based on the prevalence of PXE (approximately 1:50,000), thousands of patients with PXE worldwide may be at risk for worsening of PXE as a result of warfarin therapy.
The pathological processes of ectopic mineralization have been linked to a number of diverse clinical conditions, including such major causes of morbidity and mortality as atherosclerosis, arteriosclerosis, cancer, and immunological diseases.1–3 For example, a recent study examined the risk of death associated with coronary artery calcification in a cohort of 25,253 patients and found that coronary artery calcification was an independent risk factor for death by up to 12.5-fold.3,4 Ectopic calcification processes involving peripheral connective tissues, particularly in the skin and in arterial blood vessels, can result from two types of processes. Metastatic calcification is associated with elevated circulating levels of calcium and/or phosphate, as in chronic renal failure, whereas dystrophic calcification is secondary to a form of insult to the tissue, such as in autoimmune diseases and cancer. Metastatic calcinosis in the skin can be characterized clinically by subcutaneous nodular mineral deposits that often occur in periarticular distribution and are reversible on correction of the calcium and/or phosphate abnormalities.5 It is also observed in association with vascular calcification, as in calciphylaxis, which has a very high mortality rate.6 Dystrophic calcification occurs frequently in previously damaged or diseased tissue, and localized involvement occurs in many cutaneous inflammatory lesions as well as in benign and malignant neoplasms.1 Dystrophic calcification occurs without calcium or phosphate abnormalities at plasma levels.
A number of Mendelian genetic disorders share phenotypic similarities with the acquired forms of metastatic and dystrophic calcification. Ectopic mineralization, particularly affecting the skin and cardiovascular tissues, has been linked to mutations in familial tumoral calcinosis (both normophosphatemic and hyperphosphatemic variants),5 generalized arterial calcification of infancy,7 and CD73 deficiency.8 The prototype of heritable ectopic mineralization disorders is pseudoxanthoma elasticum (PXE), a multisystem disorder with clinical manifestations primarily in the skin, the eyes, and the cardiovascular system.9,10 A characteristic feature of PXE is late-onset but progressive mineralization of connective tissues, primarily the elastic structures in the affected organs. Skin findings consist of yellowish papules that tend to coalesce into plaques of inelastic and leathery skin at predilection sites, such as the side of the neck and flexural areas of the arms and legs.9 Diagnostic histopathology demonstrates accumulation of pleiomorphic elastotic structures in mid-dermis with mineral deposits consisting of hydroxyapatite. The skin findings, although primarily of cosmetic concern, predict development of ocular and vascular complications. The characteristic finding in the eyes is the presence of angioid streaks due to breaks in pathologically calcified Bruch’s membrane, an elastin-rich sheath behind the retina.11 These breaks result in neovascularization and bleeding into the eyes, leading to loss of visual acuity and occasionally to blindness. The cardiovascular complications are a result of mineralization of midsize arteries, leading to clinical presentations with renovascular hypertension, intermittent claudication, occasional hemorrhage from gastric arteries, early myocardial infarcts, and stroke.12
The classic form of PXE is caused by mutations in the ABCC6 gene, which encodes a transmembrane efflux transporter expressed primarily in the liver and the kidneys. Close to 600 distinct inactivating mutations in this gene have been identified in families with PXE, representing more than 1000 mutant alleles, and molecular genetics findings have clearly established that PXE is an autosomal recessive disorder.10,13,14 The precise pathomechanistic pathways leading from mutations in the ABCC6 gene (expressed primarily in the liver) to ectopic mineralization of peripheral connective tissues are currently unknown, and the nature of the substrate or substrates transported by ABCC6 under physiological conditions remains to be disclosed.15 It has been suggested that PXE is a metabolic disorder and that, in the absence of functional ABCC6 transporter activity, the circulation becomes devoid of factors that are required under normal homeostatic conditions to prevent local precipitation of calcium and phosphate.10,15 Matrix Gla protein (MGP), a powerful local antimineralization factor, has been proposed to play a role in PXE.16,17 Specifically, MGP needs to be activated by γ-glutamyl carboxylase, a vitamin K–dependent enzyme that converts Gla proteins to their γ-glutamyl carboxylated forms.18 Based on these and related clinical observations, it has been suggested that vitamin K or one of its derivatives is physiologically transported from the liver by ABCC6 into the circulation, and that, in the absence of a critical vitamin K cofactor, γ-glutamyl carboxylation of MGP does not proceed in peripheral tissues, thus allowing progressive mineralization to ensue.19
Much of our understanding of the pathological nature of PXE has been derived from an animal model, the Abcc6−/− mouse, developed by targeted ablation of the mouse homolog to ABCC6.20,21 These knockout mice recapitulate the genetic, histopathological, and ultrastructural features of PXE; specifically, they develop mineral deposits in the same organs (ie, the skin, the eyes, and the arterial blood vessels) as in patients with PXE. The mineralization process in the traditional Abcc6−/− mouse (Abcc6tm1JfK), which was initially developed on a mixed 129S1/SvImJ and C57BL/6J background and fed a standard laboratory mouse diet, ensues at approximately 5 to 6 weeks of age.20 The first site of mineralization is the connective tissue sheath of vibrissae in the muzzle skin, and quantitative assessment of the mineral deposits either by direct chemical assay of calcium and phosphorus, by computerized morphometric analysis of histopathological sections, or by small-animal computed tomography (CT), serves as a biomarker reflecting the progress of overall mineralization in these mice.22,23 Recent studies have also indicated that changing the genetic background of these mice or altering the mineral content of the diet can modify the rate of onset and the severity of mineralization.24–26
Warfarin, a powerful oral anticoagulant, inhibits γ-glutamyl carboxylation of Gla proteins, such as coagulation factors and MGP, by interfering with the vitamin K cycle. Vitamin K exists in three principal forms, in addition to some intermediate unstable forms: vitamin K (quinone), vitamin KH2 (hydroquinone), and vitamin KO (epoxide).18 The reduced vitamin KH2 serves as a cofactor for γ-glutamyl carboxylase, resulting in KO as the end product of carboxylation. The KO form is then reduced back to KH2 by enzymatic reactions involving vitamin K epoxide reductase and quinone reductase, enzymes that are inhibited by warfarin. Warfarin treatment thus results in accumulation of KO, and the reduction of KH2 is accompanied by undercarboxylation of Gla proteins, including coagulation factors in the liver and MGP in the peripheral tissues.
One of the adverse effects of warfarin is cardiovascular calcification; specifically, there is an increased prevalence of mitral and aortic valve calcification, as well as mitral annular calcium deposition, in patients with nonvalvular atrial fibrillation treated with warfarin.27,28 Furthermore, studies using a number of different animal models, and particularly rat models, have demonstrated that warfarin can elicit vascular mineralization.29–31 Considering the relationship of warfarin and vascular calcification, we hypothesized that warfarin may accelerate ectopic tissue mineralization in PXE, with clinical consequences. This hypothesis was based in part on early report that high doses of warfarin can cause focal calcification of elastic lamellae in rat arteries.30 In the present study, we developed a novel mouse model in which the effects of warfarin on ectopic mineralization were tested on an Abcc6−/− background.
Materials and Methods
Mice
The Abcc6tm1JfK mouse, a model for PXE, was developed by targeted ablation of the Abcc6 gene.20 Abcc6 wild-type (Abcc6+/+) and knockout (Abcc6−/−) mice were made congenic by backcrossing heterozygous (Abcc6+/−) mice on a C57BL/6J background for 10 generations. Mice were maintained under standard conditions at the Animal Facility of Thomas Jefferson University. All protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Proper handling and care were practiced according to the animal welfare policies of the Public Health Service.
Experimental Design and Diets
Mice were placed on specific diets at 6 weeks of age and kept on the same diet for 29 weeks (set 1, Abcc6+/+ and Abcc6−/− mice) or for 8 weeks (set 2, Abcc6−/− mice). Groups of mice in the two sets are characterized by genotype, experiment duration, and diet in Table 1.
Table 1.
Experimental Groups of Abcc6+/+ and Abcc6−/− Mice by Genotype and Diet
| Group | Genotype | n | Diet† |
|---|---|---|---|
| Set 1 (29 weeks of follow-up)∗ | |||
| A | Abcc6+/+ | 8 | Control |
| B | Abcc6+/+ | 9 | Control + W/K1 |
| C | Abcc6−/− | 8 | Control |
| D | Abcc6−/− | 9 | Control + W/K1 |
| Set 2 (8 weeks of follow-up)∗ | |||
| E | Abcc6−/− | 8 | Control |
| F | Abcc6−/− | 5 | Control + W/K1 |
| G | Abcc6−/− | 7 | Acceleration |
| H | Abcc6−/− | 9 | Acceleration + W/K1 |
Mice 6 weeks of age at the start of experiments.
Acceleration, a diet low in magnesium and enriched in phosphate; control, a standard murine laboratory diet; + W/K1, warfarin and vitamin K1 supplementation.
In set 1, Abcc6+/+ and Abcc6−/− mice were fed either laboratory autoclavable meal rodent diet 5010 (PMI Nutrition International, Brentwood, MO) (control diet; n = 8 per group) or the same diet supplemented with 3 mg warfarin and 1.5 mg vitamin K1 per gram of food (W/K1 diet; n = 9 per group). A 2-mm muzzle skin biopsy was performed 3 months after initiation of diets and every few weeks thereafter, to monitor vibrissae mineralization. After 29 weeks, the mice were euthanized and necropsied for additional analysis.
In set 2, Abcc6−/− mice were given one of four diets: control diet (n = 8), as in set 1; W/K1 diet (n = 5), as in set 1; acceleration diet (rodent diet TD.00442; Harlan Teklad, Madison, WI) (n = 7); or acceleration diet supplemented with 3 mg warfarin and 1.5 mg vitamin K1 per gram of food (acceleration + W/K1 diet; n = 9). After 8 weeks on a specific diet, two mice from each group were imaged by CT for evidence of mineralization. All mice were then euthanized and necropsied for further analysis.
Histopathological Analysis
Biopsies from muzzle skin containing vibrissae and from internal organs were fixed in 10% phosphate-buffered formalin and embedded in paraffin. The tissues were sectioned (6 μm thick), placed onto slides, and stained with H&E, Alizarin Red, or von Kossa using standard procedures. Slides were examined under light microscopy for mineralization.
Quantitation of Tissue Mineralization by Computerized Morphometric Analysis
Computerized morphometric analysis of mineralization was performed on H&E-stained sections with a Nikon (Tokyo, Japan) Te2000 microscope and an AutoQuant imaging system (AutoQuant Imaging, Watervliet, NY). The area of mineralization in vibrissae was expressed as a percentage of the total area of vibrissae per mouse, and the average percentage of mineralization was determined for each group. All images were analyzed with Image-Pro Plus software version 6.1 (Media Cybernetics, Rockville, MD).
Chemical Quantification of Calcium and Phosphate Deposition
To quantify the mineral deposition in vibrissae, muzzle skin was harvested and decalcified with 0.15 mol/L HCl for 48 hours at room temperature, and solubilized calcium and phosphate contents were determined. Calcium and phosphate levels in serum samples were also determined. Colorimetric analysis by the o-cresolphthalein complexone method [calcium (CPC) LiquiColor; Stanbio Laboratory, Boerne, TX] was performed to measure calcium content. Phosphate content was determined with a Malachite Green phosphate assay kit (BioAssay Systems, Hayward, CA). A Bio-Rad model 680 microplate reader (Bio-Rad Laboratories, Hercules, CA) was used to obtain absorbance values of samples. The filter was set at 595 nm for calcium and 656 nm for phosphate measurements. Values were normalized to tissue weight.
Immunofluorescence and Immunohistochemistry
Paraffin-embedded tissue sections (6 μm thick) were deparaffinized in xylene and rehydrated in descending concentrations of ethanol. Tissues were subject to antigen unmasking by boiling in citrate-based unmasking solution (Vector Laboratories, Burlingame, CA) for 15 minutes and then permeabilized with 0.1% Triton X-100 in PBS. Slides were washed in 0.1% Tween 20 in PBS (PBST), and sections were blocked with 3% bovine serum albumin in PBST for 1 hour at room temperature. After blocking, sections were incubated with primary antibody against α-fetuin (1:100; R&D Systems, Minneapolis, MN) or osteopontin (1:20; R&D Systems) overnight at 4°C. Negative controls were incubated with 3% bovine serum albumin in PBST in place of the primary antibody. Slides were washed again in PBST and incubated with Alexa Fluor 594 anti-goat IgG secondary antibody (1:800; Life Technologies-Invitrogen, Carlsbad, CA) for 1 hour. Fluorescence microscopy was immediately performed using a Nikon Te2000 microscope.
Immunohistochemical staining was performed on paraffin-embedded tissue sections and stained with either one of two monoclonal MGP antibodies (Vascular Products, Maastricht, The Netherlands). Briefly, sections were heated in 0.2% (w/v) citric acid at pH 6.0 for 15 minutes before a PBS wash and incubation with MGP antibodies. Anti-cMGP (1 μg/mL) recognizing carboxylated MGP (cMGP), and anti-ucMGP (1 μg/mL) recognizing uncarboxylated MGP (ucMGP), respectively, were diluted in blocking reagent (Roche Diagnostics, Indianapolis, IN; Mannheim, Germany). Negative controls were performed by either omitting the primary antibody or by substitution for an irrelevant antibody. Biotinylated sheep anti-mouse IgG (Amersham; GE Healthcare, Little Chalfont, UK) was used as a second antibody, followed by incubation with avidin-linked alkaline phosphatase complex (Dako, Glostrup, Denmark), and then by staining with the alkaline phosphatase kit I (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin and mounted with Imsol mounting medium (Klinipath, Duiven, The Netherlands) and Entellan rapid mounting medium (Merck Millipore, Darmstadt, Germany).
Blood INR Measurements
Blood was collected in test tubes containing 3.2% buffered sodium citrate (Medicago, Uppsala, Sweden), with a 9:1 volume ratio of blood to citrate buffer. Measurements of international normalized ratio (INR) values and prothrombin time were performed on an analyzer at the Cardeza Special Hemostasis Laboratory of Thomas Jefferson University.
Measurement of Warfarin and K1 Intake
The serum concentration of K1 and KO was determined using a modified high-performance liquid chromatography method with postcolumn chemical reduction and fluorescence detection, based on the method described by Davidson and Sadowski.32 The lower limit of quantification for this assay was typically 0.05 μg/L. Warfarin and the assay internal standard (acenocoumarin) were extracted from serum into methyl tert-butyl ether after acidification with HCl. After centrifugation, the ethereal phase was concentrated and subsequently reconstituted in mobile phase and injected into the high-performance liquid chromatography column with detection by UV absorbance (270 nm and 310 nm). Serum concentrations were determined from the linear calibration plots (peak height ratios of warfarin to acenocoumarin against the equivalent weight ratios) obtained on direct injection of pure standards containing warfarin and acenocoumarin at different weight ratios.
EDAX and Topographic Mapping
Sections of muzzle skin containing mineral deposits were analyzed by energy dispersive X-ray (EDAX) analysis and topographic mapping. Paraffin sections were mounted onto carbon carriers. Specimens were imaged and analyzed for elemental composition with a JEOL-T330A scanning electron microscope (JEOL, Tokyo, Japan) fitted with an EDAX microanalysis analyzer. X-ray topographic maps of calcium and phosphorus were acquired using NSS software, version 2.3 (Thermo Fisher Scientific, Swedesboro, NJ).
Small-Animal CT
After 8 weeks, two mice each from groups E to H (control diet, W/K1 diet, acceleration diet, and acceleration + W/K1 diet) were examined for mineralization by CT scanning. Mice were anesthetized with a xylazine–ketamine–acetopromazine cocktail (160 μL per 25 g body weight of 10 mg/kg xylazine, 200 mg/kg ketamine, 2 mg/kg acetopromazine) and then scanned in a MicroCAT II system (ImTek, Oak Ridge, TN). To analyze mineralization in vibrissae, a three-dimensional facial rendering was done for each mouse using Amira version 3.1 software (Visualization Sciences Group, Burlington, MA).
Clinical Survey
Of the approximately 4000 patients enrolled into the PXE International registry, 1747 patients for whom an e-mail address was available were surveyed by e-mail regarding present or past use of anticoagulants. Of the 539 respondents, 93 indicated present or past use of anticoagulation therapy. These patients were sent a detailed questionnaire regarding the type and length of anticoagulation therapy and clinical progression of their PXE disease while on this therapy. This survey was approved by the Genetic Alliance Institutional Review Board.
Statistical Analysis
Student’s t-test was used to compare quantitative results of morphometric analysis, calcium and phosphorus chemical assay, and measurement of blood INR, warfarin, and vitamin K1 intake. Fisher’s exact test was used to determine the difference between proportions of mineralization in organs of mice fed with different diets.
Results
Development of a Novel Warfarin-Induced Ectopic Mineralization Mouse Model
Previous studies have demonstrated that feeding rats with warfarin can result in early vascular calcification.29–31 Also, feeding of ApoE−/− knockout mice with a warfarin-containing diet has been shown to accelerate atherosclerotic calcification.33 There are no reports, however, on long-term feeding of normolipidemic mice with warfarin. We therefore placed Abcc6+/+ and Abcc6−/− mice on a standard rodent diet without (control diet) or with warfarin supplementation (W/K1 diet) at 6 weeks of age, after weaning, at the time just before the Abcc6−/− mice develop overt mineralization of the dermal sheath of vibrissae and of cardiovascular tissues. All the warfarin-containing diets were supplemented with vitamin K1, a strategy that prevents the warfarin-induced inhibition of the vitamin K cycle in the liver and therefore does not affect the γ-glutamyl carboxylation of the coagulation factors. Thus, these mice do not suffer from excessive bleeding.29
Two experimental designs were used, as described under Materials and Methods, in eight groups of mice (Table 1). In the first set of experiments, 6-week-old mice of both genotypes were placed on the control or the W/K1 diet and were monitored for 29 weeks. At this point, the mice were sacrificed, subjected to blood analysis, and examined for evidence of ectopic mineralization. Testing of plasma in the mice fed the W/K1 diet detected significant levels of warfarin, up to 12 mg/L, whereas warfarin levels in the mice fed the control diet were below the detection limit (<0.12 mg/L) (Table 2). The mice fed the W/K1 diet also exhibited significantly elevated (up to 200-fold) levels of vitamin K1 (K1 + KH2), compared with wild-type mice on the control diet. Importantly, significant levels of KO were detected in serum of mice fed the W/K1 diet (up to ∼300 μg/L), whereas those on the control diet showed values below the detection limit (<0.12 μg/L), indicating that the warfarin treatment resulted in accumulation of KO due to inhibition of the enzymatic reduction of KO to KH2 (Table 2). Although these results confirmed that warfarin effectively inhibited the vitamin K cycle, the expected inhibition of coagulation factors in the liver was apparently overcome by addition of vitamin K1 to the diet. Specifically, the INR and prothrombin time, which reflect blood coagulation, were not altered in mice fed the experimental diet, compared with mice fed the control diet (data not shown). Finally, it should be noted that the serum calcium concentration was slightly elevated in mice fed the W/K1 diet, but the serum phosphorus levels and the accompanying Ca/P ratio were not altered significantly (Table 3).
Table 2.
Plasma Warfarin and Serum Vitamin K1 Concentrations in Abcc6+/+ and Abcc6−/− Mice on Control or W/K1 Supplementation Diet
| Group | Warfarin (mg/L) | Vitamin K1 |
|
|---|---|---|---|
| K1+KH2 (μg/L) | KO (μg/L) | ||
| A | BLD | 0.57 ± 0.05 | BLD |
| B | 12.04 ± 6.96∗ | 97.30 ± 26.97∗ | 308.41 ± 116.41∗ |
| C | BLD | 0.71 ± 0.07 | BLD |
| D | 7.27 ± 2.28∗∗ | 51.99 ± 13.01∗∗ | 241.05 ± 32.58∗∗ |
Detection limits for K (K1+KH2) and KO are 0.5 μg/L and 0.12 μg/L, respectively. Detection limit for warfarin is 0.12 mg/L. Data are expressed as means ± SEM. n = 8 (groups A and C, control diet) or 9 (groups B and D, W/K1 supplementation diet).
∗P < 0.05 versus group A (Abcc6+/+, control diet), ∗∗P < 0.01 versus group C (Abcc6−/−, control diet).
BLD, below limit for detection.
Table 3.
Calcium and Phosphorus Concentrations in the Serum of Abcc6+/+ and Abcc6−/− Mice on Control or W/K1 Supplementation Diet
| Parameter | Serum concentration |
|||
|---|---|---|---|---|
| A | B | C | D | |
| Calcium (mg/dL) | 10.69 ± 0.12 | 11.63 ± 0.35∗ | 10.71 ± 0.16 | 11.71 ± 0.23† |
| Phosphorus (mg/dL) | 10.85 ± 0.44 | 11.51 ± 0.80 | 9.68 ± 0.79 | 10.97 ± 0.51 |
| Ca/P ratio | 1.00 ± 0.04 | 0.97 ± 0.04 | 0.91 ± 0.07 | 0.94 ± 0.04 |
Data are expressed as means ± SEM. n = 8 (groups A and C, control diet) or 9 (groups B and D, W/K1 supplementation diet).
P < 0.05 versus group A (Abcc6+/+, control diet).
P < 0.01 versus group C (Abcc6−/−, control diet).
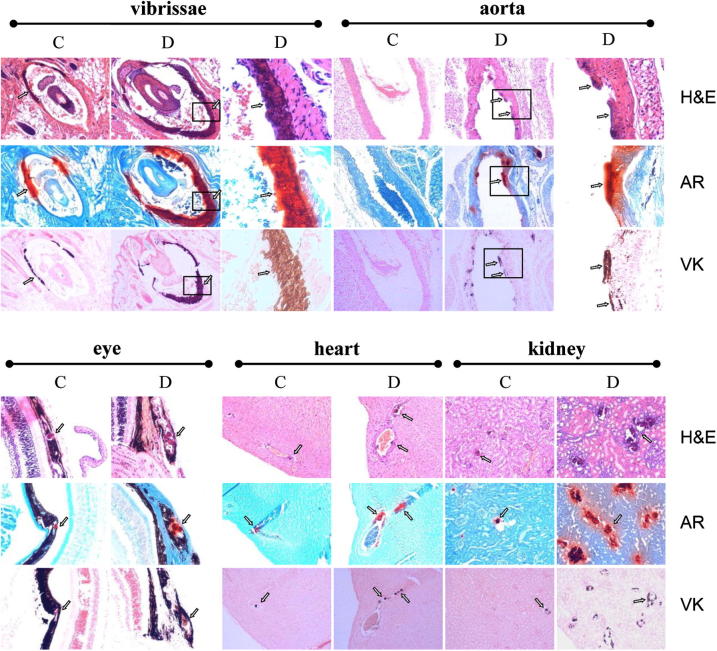
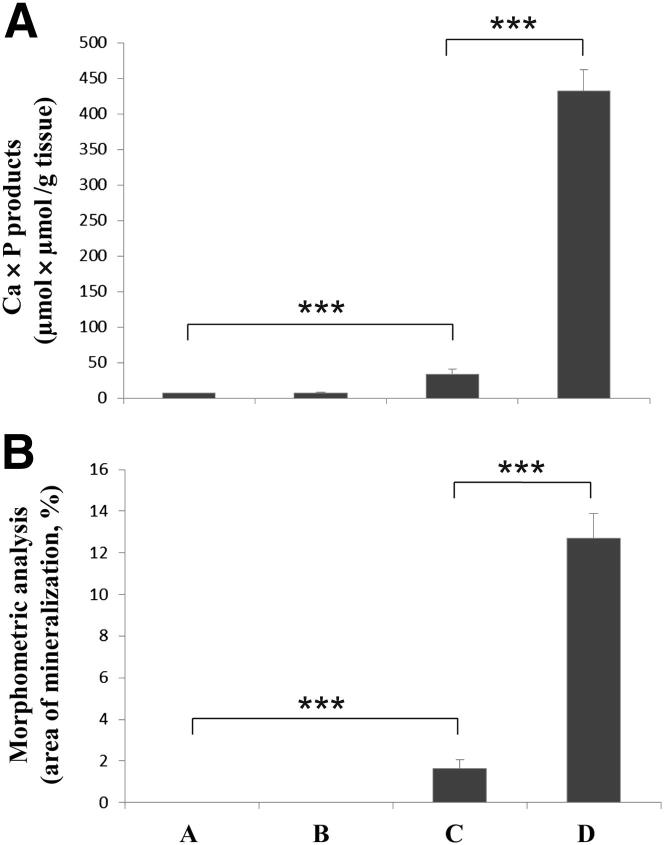
Histopathological examination of Abcc6+/+ mice placed on the W/K1 diet for 29 weeks (group B) revealed minimal evidence of ectopic mineralization in the dermal sheath of vibrissae and in the eyes (one mouse out of nine examined), whereas the wild-type mice kept on the control diet (group A) showed no evidence of soft tissue mineralization in the vibrissae, kidneys, heart, aorta, or eyes (Table 4). Examination of Abcc6−/− mice kept on the control diet (group C) demonstrated significant mineralization in the dermal sheath of vibrissae, kidneys, heart, and eyes, consistent with previous report20 (Table 4). However, Abcc6−/− mice on the warfarin-supplemented diet (group D) exhibited a markedly increased degree of mineralization, and each of the nine mice examined showed mineral deposits in vibrissae, kidneys, heart, aorta, and eyes (Figure 1 and Table 4). The degree of mineralization was quantitated by chemical and morphometric analysis of muzzle skin containing the vibrissae. The results, both from direct assay of calcium and phosphate content of the skin and from computerized morphometric analysis of histopathological sections, revealed up to 16-fold increases in ectopic mineralization in Abcc6−/− mice fed the W/K1 diet, compared with Abcc6−/− mice on the control diet (Figure 2). Thus, in our mouse model, warfarin clearly increases the ectopic mineralization of peripheral connective tissues and accelerates the mineralization process elicited by Abcc6 deficiency.
Table 4.
Soft Tissue Mineralization in Abcc6+/+ and Abcc6−/− Mice Placed On Different Diets
| Group | Mineralization in soft tissues [n/N (%)] |
||||
|---|---|---|---|---|---|
| Vibrissae | Kidneys | Heart | Aorta | Eyes | |
| A | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) | 0/8 (0) |
| B | 1/9 (11) | 0/9 (0) | 0/9 (0) | 0/9 (0) | 1/9 (11) |
| C | 8/8 (100)∗ | 2/8 (25) | 3/8 (38) | 0/8 (0) | 2/8 (25) |
| D | 9/9 (100) | 9/9 (100)‡ | 9/9 (100)‡ | 9/9 (100)‡ | 9/9 (100)‡ |
| E | 8/8 (100) | 0/8 (0) | 2/8 (25) | 0/8 (0) | 1/8 (13) |
| F | 5/5 (100) | 5/5 (100)‡ | 0/5 (0) | 0/5 (0) | 3/5 (60) |
| G | 7/7 (100) | 4/7 (57)† | 6/7 (86)‡ | 0/7 (0) | 3/7 (43) |
| H | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100)§ | 3/9 (33) |
Mineralization was determined by histopathologic examination of H&E-stained tissue sections.
P < 0.01 versus group A (Abcc6+/+, control diet).
P < 0.05, ‡P < 0.01 versus group C (Abcc6−/−, control diet).
P < 0.01 versus group G (Abcc6−/−, acceleration diet without W/K1 supplementation).
Figure 1.
Enhanced mineralization in the dermal sheath of vibrissae and in the aorta, eyes, heart, and kidneys of Abcc6−/− mice fed the experimental W/K1 diet (group D), compared with Abcc6−/− mice kept on the control diet (group C). Mineral deposits (arrows) were visualized by H&E, Alizarin Red (AR), and von Kossa (VK) stains. For vibrissae and aorta, images in the right column correspond to the boxed regions in the center column, at higher magnification (×300). Original magnification: ×100 (vibrissae, aorta, heart); ×150 (eye, kidney).
Figure 2.
Quantitation of the mineral deposits in the muzzle skin containing the dermal sheath of vibrissae by direct chemical assay of calcium and phosphorus (A) and by computerized morphometric analysis of histopathological sections (B) in groups A to D (both genotypes, control and W/K1 diets). Data are expressed as means ± SEM. n = 8 (control) or 9 (W/K1). ∗∗∗P < 0.001.
Warfarin and a Diet Low in Magnesium and High in Phosphate Independently Accelerate the Mineralization Process
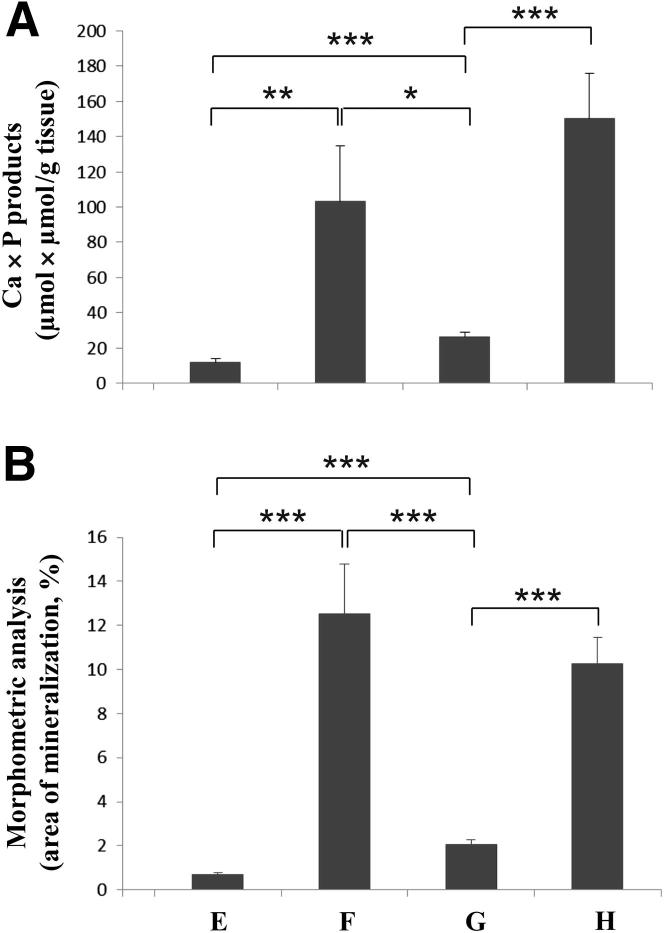
Previous studies have indicated that manipulation of the mouse diet can alter the degree of mineralization in Abcc6−/− mice. Specifically, a diet with reduced magnesium and elevated phosphate content (the so-called acceleration diet) has been shown to markedly enhance mineralization in these mice.25,26 In a second set of experiments, we examined the effect of warfarin in a short-term study in which 6-week-old Abcc6−/− mice were placed on the control diet, the W/K1 diet, the acceleration diet, or the acceleration diet + W/K1. Mice were monitored for 8 weeks, and then examined for mineralization. The degree of mineralization in the dermal sheath of vibrissae was monitored by small-animal CT scanning, which allows noninvasive evaluation of the mineralization process.23 Our results suggested a minimal degree of mineralization in Abcc6−/− mice at 8 weeks on the control diet (group E), but this process was significantly accelerated by the addition of warfarin to the diet (group F). Abcc6−/− mice kept on the acceleration diet for 8 weeks (group G) showed evidence of significant mineralization in the dermal sheath of vibrissae, and addition of warfarin to the diet resulted in massive mineralization (group H) (Figure 3).
Figure 3.
Noninvasive evaluation of the mineralization process in dermal sheath of vibrissae by micro-CT scan. The presence of mineral deposits (arrows) is revealed in single slices (bottom row). Three-dimensional reconstruction (top row) demonstrates the presence of mineral deposits in the vibrissae (arrowheads), particularly in Abcc6−/− mice fed the acceleration diet (group G) or the acceleration diet + W/K1 (group H). No mineralization of dermal sheath of vibrissae was noted in either wild-type or knockout mice kept on the control diet (groups A and E, respectively). The Abcc6−/− mice on the W/K1 diet (group F) showed extensive mineralization.
Histopathological examination of the Abcc6−/− mice by routine H&E staining for calcification, as well as by Alizarin Red and von Kossa staining, revealed marked mineralization of the dermal sheath of vibrissae in all mice in set 2 (groups E–H), regardless of diet (Table 4). However, mice kept on the control diet (group E) showed only partial mineralization in the heart and eyes (25% and 13% of animals, respectively), and no mineralization was noted in the kidneys and aorta. Supplementation of the control diet with warfarin and vitamin K1 (group F) increased the mineralization noted in kidneys to 100% of the five animals examined (Table 4). The mice kept on the acceleration diet (group G) exhibited extensive mineralization in the kidneys, heart, and eyes. The degree of mineralization with the acceleration diet was further increased by W/K1 supplementation (group H). All group H mice exhibited mineral deposits in the dermal sheath of vibrissae, the kidneys, the heart, and aorta (Table 4). The histologically observed increases in the mineral content were further confirmed by direct calcium and phosphate chemical measurements, and by computerized morphometric analysis of the dermal sheath of vibrissae (Figure 4).
Figure 4.
Quantitation of mineralization in dermal sheath of vibrissae of Abcc6−/− mice by direct chemical assay of calcium and phosphorous (A) and by computerized morphometric analysis of histopathological sections (B) in groups E to H. Data are expressed as means ± SEM. n = 5 (W/K1), 7 (acceleration), 8 (control), or 9 (acceleration + W/K1). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Characterization of Mineral Deposits
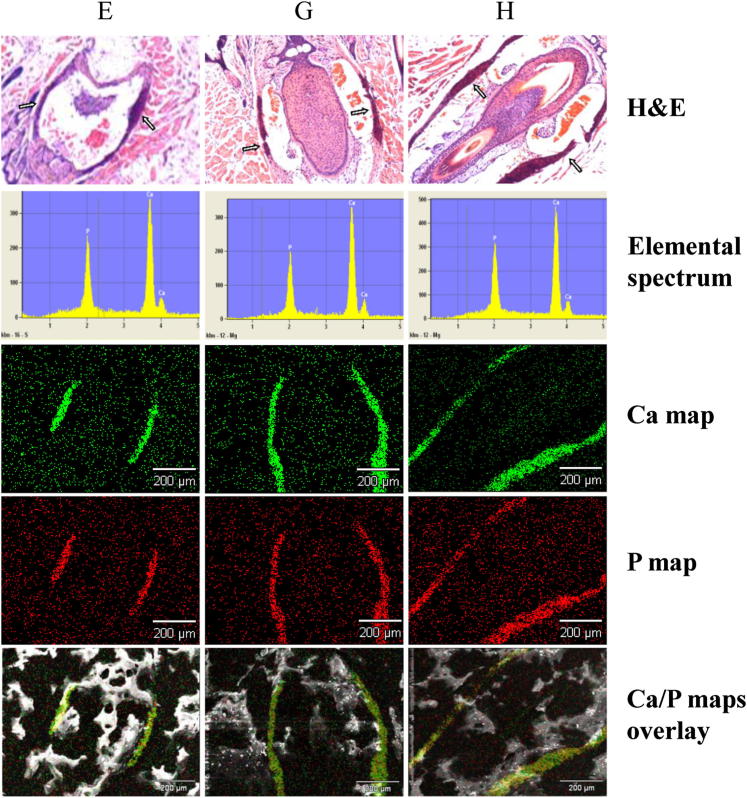
We have previously demonstrated that the mineral deposits found in the dermal sheath of vibrissae of Abcc6−/− mice kept on a standard laboratory diet consist of calcium hydroxyapatite, with a calcium/phosphate ratio of approximately 2.0:1, consistent with hydroxyapatite in endochondral bone.34 In the present study, we used energy dispersive X-ray (EDAX) analysis with topographic (RADAR) mapping capabilities to examine whether W/K1 supplementation of the acceleration diet changes the composition of the mineral deposits. The results indicated that, in Abcc6−/− mice fed the acceleration diet without or with W/K1 supplementation (group G and H, respectively), the principal components of the mineral deposits were calcium and phosphorus, in a ratio of approximately 2.0:1; this is similar to findings in Abcc6−/− mice kept on the control diet (group E) (Figure 5). We further demonstrated colocalization of calcium and phosphorus in these deposits by topographic mapping (Figure 5), suggesting that the mineral deposits consist of hydroxyapatite, the principal mineral component of bone.
Figure 5.
Analysis of the mineral content in deposits in dermal sheath of vibrissae of Abcc6−/− mice on the control, acceleration, or acceleration + W/K1 diet (groups E, G, and H, respectively). Mineral deposits identified in H&E-stained sections (arrows, top row) were subjected to EDAX analysis. The elemental spectrum (row 2) revealed the presence of calcium and phosphorus as the principal ions in approximately a 2.0:1 ratio in all samples. Topographic (RADAR) mapping identified calcium and phosphorous (rows 3 and 4) in a colocalization distribution, as shown by overlay maps (bottom row). Scale bar = 200 μm. Original magnification (H&E stain), ×100.
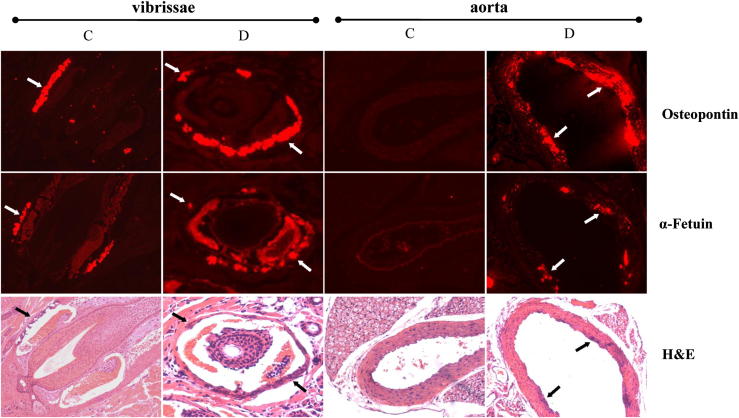
To further explore the nature of the mineralization processes in this novel mouse model, the expression of two mineralization-associated proteins, osteopontin and α-fetuin, was examined in the dermal sheath of vibrissae depicting ectopic mineralization of Abcc6−/− fed the control diet (group C) or the W/K1 diet (group D). Immunofluorescence revealed increased presence (in group D, compared with the control, group C) of both osteopontin and α-fetuin, colocalized with the mineralized areas, and the amount of protein, as judged semiquantitatively by the immunofluorescence signal, corresponded to the degree of mineralization (Figure 6). In aorta, similar changes were present in group D (Figure 6). Thus, the increase in mineralization, which has previously been shown to reflect increased mineral-to-matrix ratio as determined by Fourier transform infrared imaging spectroscopy,34 suggests that there is a progressive maturation of the mineral deposits to form hydroxyapatite, and that this process is accelerated independently by Abcc6 deficiency, altered magnesium and phosphate content of the diet, and dietary warfarin.
Figure 6.
Immunological demonstration of osteopontin (top row) and α-fetuin (middle row) associated with mineral deposits (arrows) in the dermal sheath of vibrissae and aorta in Abcc6−/− mice fed the control diet (group C) or the W/K1 diet (group D). Mineral deposits (arrows, bottom row) were visualized by H&E. Original magnification, ×100.
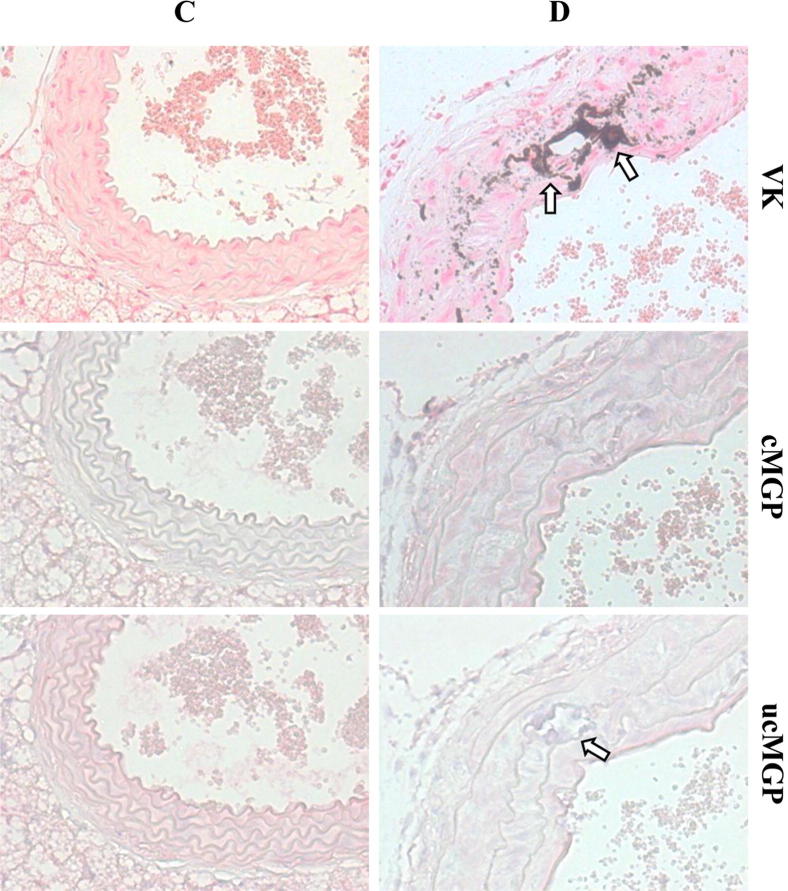
Evidence for the Pathomechanistic Role of MGP
Previous studies have suggested that the degree of γ-carboxylation of MGP due to altered vitamin K status may play a role in ectopic mineralization in Abcc6−/− mice.10,19 We therefore postulated that warfarin in diet of Abcc6−/− mice might further increase mineralization by preventing γ-glutamyl carboxylation of MGP. Tissue sections of aorta were stained with an antibody specifically recognizing the uncarboxylated form of MGP (ucMGP) and an antibody recognizing the carboxylated form of MGP (cMGP). The results suggested a greater abundance of ucMGP than cMGP in Abcc6−/− mice fed on the W/K1 diet (Figure 7). This finding is consistent with recent demonstration of decreased expression of cMGP in the plaque of ApoE−/− mice fed with warfarin.33
Figure 7.
Analysis of cMGP and ucMGP in aorta of Abcc6−/− mice kept on the control diet (group C) or the W/K1 diet (group D). Note mineralization in von Kossa (VK)–stained sections (arrows). There was a greater abundance of ucMGP (arrow) than cMGP in group D. Original magnification, ×300.
Clinical Perspective
To explore the clinical perspective of our findings on warfarin-induced acceleration of mineralization in Abcc6−/− mice, we analyzed the patient database maintained by PXE International, the premier patient advocacy organization for this disease.35 The entire patient registry consists of approximately 4000 individuals with PXE. A total of 1747 patients with PXE and with known e-mail addresses were approached with a questionnaire as to their current or past anticoagulation therapy (blood thinners), with specific emphasis on warfarin (Coumadin). Of the 539 individuals who replied, 93 indicated present or past use of anticoagulation therapy for various clinical indications. These respondents were sent a detailed questionnaire regarding the type and length of anticoagulation therapy and clinical progression of their disease while on this therapy. Examination of the detailed drug history revealed that 13 individuals specifically reported warfarin use for an average of 4.05 ± 3.41 years (means ± SD; range, 0.08–14). Thus, a total of 2.4% of PXE patients who responded to the initial inquiry were identified as past or present users of warfarin, possibly being at risk for accelerated mineralization.
Discussion
Pseudoxanthoma elasticum is a heritable mineralization disorder with considerable morbidity and mortality. The characteristic feature of this disease is ectopic mineralization of peripheral connective tissues with primary clinical manifestations in the skin, the eyes, and the cardiovascular system.9,10 The classic form of PXE is caused by mutations in the ABCC6 gene, which is expressed primarily in the liver and the kidneys.10,36,37 PXE has been suggested to be a metabolic disorder at the interface of genome and environment.38 Although the pathomechanistic details leading from mutations in the ABCC6 gene into tissue mineralization are currently unknown,15 it is clear that the clinical manifestations are primarily due to ectopic mineralization, manifesting in the skin as yellowish papules and inelastic skin, in the eyes as angioid streaks due to breakage in mineralized Bruch’s membrane, and in arterial blood vessels due to mineralization of elastic lamina. Thus, factors that modulate the extent of mineralization are expected to have significant effects on the severity of the disease and development of complications.
In the present study, we have demonstrated that warfarin, a commonly used oral anticoagulant, has a major effect on the degree of mineralization in Abcc6−/− mice, which serve as a model system for PXE. These mice were fed a diet supplemented with warfarin and vitamin K1, a combination that has been shown to result in vascular mineralization in rats.29 Warfarin elicits its anticoagulation effects by preventing the physiological activation of vitamin K–dependent coagulation factors in the liver by γ-glutamyl carboxylation, an enzymatic reaction requiring vitamin K as a cofactor. However, the experimental W/K1 diet bypasses the inhibition of γ-glutamyl carboxylation in the liver,29 and consequently the mice do not develop a coagulation disorder (as was demonstrated in the present study by normal INR and prothrombin time measurements). It is expected, however, that γ-glutamyl carboxylation of Gla protein, such as MGP, in the peripheral tissues is inhibited by warfarin, a finding consistent with the presence of ucMGP in peripheral connective tissues of our mice. Thus, our results suggest that lack of activated MGP, which physiologically serves as a powerful, local antimineralization factor,39 is responsible for the profound tissue mineralization exhibited by mice fed the W/K1 diet.
Demonstration that warfarin increases the mineralization has both pathomechanistic and clinical implications with respect to understanding of various facets of PXE. First, MGP has been suggested to play a role in PXE, based on demonstration of uncarboxylated forms of MGP in tissues of Abcc6−/− mice kept on a standard diet, as well as in skin biopsies from patients with PXE.16,17 It has been suggested that Abcc6 serves in the liver as an efflux pump transporting vitamin K or one of its derivatives, such as glutathione conjugate (vitamin K3–GSH), to the circulation.19 Thus, in the absence of functional Abcc6 pump activity, the peripheral tissues may become deficient in vitamin K, resulting in undercarboxylation of MGP. This hypothesis has been recently tested in studies in which vitamin K1 or K2 was supplemented in high doses in the diet of Abcc6−/− mice.40–42 Although dietary supplementation of vitamin K resulted in significant increases in the serum vitamin K levels in these studies, no effect on connective tissue mineralization was observed. Similarly, intravenous infusion of vitamin K3–GSH did not affect the development of mineral deposits in these mice.40 Thus, the results indicate that mineralization in Abcc6−/− mice is not simply a direct result of vitamin K deficiency. In this context, it should be noted that the serum vitamin K levels have been suggested to be somewhat reduced in patients with PXE, perhaps providing a contributory factor to the mineralization process.43
Our demonstration that the experimental diet resulted in extensive mineralization in Abcc6−/− mice suggests that, in the case of PXE, the inhibition of γ-glutamyl carboxylation of MGP is incomplete, and that reduction of KH2 by warfarin as a result of interference with the vitamin K cycle synergistically accelerates this process. An alternative explanation is that ectopic mineralization in Abcc6−/− mice and in patients with PXE is the result of a process completely independent of the γ-glutamyl carboxylation status of MGP, and that treatment of these mice with warfarin has an additive, independent effect.
Our observations may also have critical implications for the clinical management of patients with PXE. Specifically, it should be noted that warfarin treatment of patients with no evidence of PXE has been reported to result in cardiovascular mineralization.27,28,44–46 If the percentage of PXE patients found in our survey to be warfarin users is representative of the global setting, then thousands of patients worldwide may be at risk for increased ectopic mineralization and severity of PXE as a result of anticoagulant therapy. Anecdotally, many of the warfarin users in our survey reported worsening of their clinical signs and symptoms while on anticoagulation therapy; however, direct association of warfarin intake and progression of their clinical disease could not be established in our cohort because PXE is a slowly progressive condition with a variable and often unpredictable course.9
In addition to PXE, other heritable disorders manifest with ectopic tissue mineralization affecting skin and the cardiovascular systems. These conditions include familial tumoral calcinosis, the normophosphatemic variant being due to mutations in SAMD9 and the hyperphosphatemic variants being due to mutations in the GALNT3, FGF23, and KL genes.5 Generalized arterial calcification of infancy, caused by mutations either in the ENPP1 or the ABCC6 gene, affects primarily the arterial blood vessels, but these patients can also demonstrate PXE-like cutaneous features.7,47 Patients with CD73 deficiency due to mutations in the NT5E gene exhibit vascular mineralization, clinically similar to but histopathologically distinct from PXE.8,48 These observations suggest the presence of an intricate mineralization and antimineralization network in tissues. A careful balance of factors both promoting and preventing connective tissue mineralization at the local level is required for normal homeostasis.49 Finally, it should be noted that there are a number of acquired conditions, often associated with inflammatory reactions, that result in connective tissue mineralization.1 It is therefore conceivable that many patients with acquired or heritable disorders prone to ectopic mineralization are at risk for worsened clinical outcome as a result of anticoagulant therapy with warfarin.
Acknowledgments
We thank Alix Grand-Pierre and Dian Wang for animal care; Gerald Harrison for EDAX and RADAR mapping; Adele Donahue and Mark Pawlowski for histopathology; Megan Hevelow for blood coagulation analysis; Jocelyn Andrel and Terry Hyslop for statistical analysis; Bishnulrari Paydal, Neil Mehta, and Madhukar Thakur for CT scanning; Abbie Moore for clinical database analysis; and Carol Kelly for manuscript preparation.
Footnotes
Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01-AR28450 and R01-AR55225 to J.U.). Q.L. is recipient of a Dermatology Foundation Research Career Development Award.
Q.L. and H.G. contributed equally to this work.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the peer review process or final disposition of this article.
References
- 1.Touart D.M., Sau P. Cutaneous deposition diseases. Part II. J Am Acad Dermatol. 1998;39:527–544. doi: 10.1016/s0190-9622(98)70001-5. quiz 545–546. [DOI] [PubMed] [Google Scholar]
- 2.Köstler E., Porst H., Wollina U. Cutaneous manifestations of metabolic diseases: uncommon presentations. Clin Dermatol. 2005;23:457–464. doi: 10.1016/j.clindermatol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Budoff M.J., Shaw L.J., Liu S.T., Weinstein S.R., Mosler T.P., Tseng P.H., Flores F.R., Callister T.Q., Raggi P., Berman D.S. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 4.Rennenberg R.J., Kessels A.G., Schurgers L.J., van Engelshoven J.M., de Leeuw P.W., Kroon A.A. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130:652–660. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weenig R.H., Sewell L.D., Davis M.D., McCarthy J.T., Pittelkow M.R. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569–579. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 7.Nitschke Y., Baujat G., Botschen U., Wittkampf T., du Moulin M., Stella J., Le Merrer M., Guest G., Lambot K., Tazarourte-Pinturier M.F., Chassaing N., Roche O., Feenstra I., Loechner K., Deshpande C., Garber S.J., Chikarmane R., Steinmann B., Shahinyan T., Martorell L., Davies J., Smith W.E., Kahler S.G., McCulloch M., Wraige E., Loidi L., Höhne W., Martin L., Hadj-Rabia S., Terkeltaub R., Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Hilaire C., Ziegler S.G., Markello T.C., Brusco A., Groden C., Gill F., Carlson-Donohoe H., Lederman R.J., Chen M.Y., Yang D., Siegenthaler M.P., Arduino C., Mancini C., Freudenthal B., Stanescu H.C., Zdebik A.A., Chaganti R.K., Nussbaum R.L., Kleta R., Gahl W.A., Boehm M. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neldner K.H. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 10.Uitto J., Li Q., Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgalas I., Tservakis I., Papaconstaninou D., Kardara M., Koutsandrea C., Ladas I. Pseudoxanthoma elasticum, ocular manifestations, complications and treatment. Clin Exp Optom. 2011;94:169–180. doi: 10.1111/j.1444-0938.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 12.Utani A., Tanioka M., Yamamoto Y., Taki R., Araki E., Tamura H., Miyachi Y. Relationship between the distribution of pseudoxanthoma elasticum skin and mucous membrane lesions and cardiovascular involvement. J Dermatol. 2010;37:130–136. doi: 10.1111/j.1346-8138.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 13.Pfendner E.G., Vanakker O.M., Terry S.F., Vourthis S., McAndrew P.E., McClain M.R., Fratta S., Marais A.S., Hariri S., Coucke P.J., Ramsay M., Viljoen D., Terry P.F., De Paepe A., Uitto J., Bercovitch L.G. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringpfeil F., Lebwohl M.G., Christiano A.M., Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA. 2000;97:6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uitto J., Bercovitch L., Terry S.F., Terry P.F. Pseudoxanthoma elasticum: progress in diagnostics and research towards treatment: summary of the 2010 PXE International Research Meeting [Erratum appeared in Am J Med Genet A 2011, 155A:3176] Am J Med Genet A. 2011;155A:1517–1526. doi: 10.1002/ajmg.a.34067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheduzzi D., Boraldi F., Annovi G., DeVincenzi C.P., Schurgers L.J., Vermeer C., Quaglino D., Ronchetti I.P. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab Invest. 2007;87:998–1008. doi: 10.1038/labinvest.3700667. [DOI] [PubMed] [Google Scholar]
- 17.Li Q., Jiang Q., Schurgers L.J., Uitto J. Pseudoxanthoma elasticum: reduced gamma-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6-/-) Biochem Biophys Res Commun. 2007;364:208–213. doi: 10.1016/j.bbrc.2007.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berkner K.L. The vitamin K-dependent carboxylase. Annu Rev Nutr. 2005;25:127–149. doi: 10.1146/annurev.nutr.25.050304.092713. [DOI] [PubMed] [Google Scholar]
- 19.Borst P., van de Wetering K., Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 20.Klement J.F., Matsuzaki Y., Jiang Q.J., Terlizzi J., Choi H.Y., Fujimoto N., Li K., Pulkkinen L., Birk D.E., Sundberg J.P., Uitto J. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgels T.G., Hu X., Scheffer G.L., van der Wal A.C., Toonstra J., de Jong P.T., van Kuppevelt T.H., Levelt C.N., de Wolf A., Loves W.J., Scheper R.J., Peek R., Bergen A.A. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–1773. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Q., Li Q., Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–1402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- 23.Le Corre Y., Le Saux O., Froeliger F., Libouban H., Kauffenstein G., Willoteaux S., Leftheriotis G., Martin L. Quantification of the calcification phenotype of Abcc6-deficient mice with microcomputed tomography. Am J Pathol. 2012;180:2208–2213. doi: 10.1016/j.ajpath.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Uitto J. The mineralization phenotype in Abcc6 (-/-) mice is affected by Ggcx gene deficiency and genetic background–a model for pseudoxanthoma elasticum. J Mol Med (Berl) 2010;88:173–181. doi: 10.1007/s00109-009-0522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaRusso J., Li Q., Jiang Q., Uitto J. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−/−)) J Invest Dermatol. 2009;129:1388–1394. doi: 10.1038/jid.2008.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Q., Uitto J. Restricting dietary magnesium accelerates ectopic connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) Exp Dermatol. 2012;21:694–699. doi: 10.1111/j.1600-0625.2012.01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerner R.G., Aronow W.S., Sekhri A., Palaniswamy C., Ahn C., Singh T., Sandhu R., McClung J.A. Warfarin use and the risk of valvular calcification. J Thromb Haemost. 2009;7:2023–2027. doi: 10.1111/j.1538-7836.2009.03630.x. [DOI] [PubMed] [Google Scholar]
- 28.Chatrou M.L., Winckers K., Hackeng T.M., Reutelingsperger C.P., Schurgers L.J. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012;26:155–166. doi: 10.1016/j.blre.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Schurgers L.J., Spronk H.M., Soute B.A., Schiffers P.M., DeMey J.G., Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- 30.Price P.A., Faus S.A., Williamson M.K. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 31.Howe A.M., Webster W.S. Warfarin exposure and calcification of the arterial system in the rat. Int J Exp Pathol. 2000;81:51–56. doi: 10.1046/j.1365-2613.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson K.W., Sadowski J.A. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–421. doi: 10.1016/s0076-6879(97)82124-6. [DOI] [PubMed] [Google Scholar]
- 33.Schurgers L.J., Joosen I.A., Laufer E.M., Chatrou M.L., Herfs M., Winkens M.H., Westenfeld R., Veulemans V., Krueger T., Shanahan C.M., Jahnen-Dechent W., Biessen E., Narula J., Vermeer C., Hofstra L., Reutelingsperger C.P. Vitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS One. 2012;7:e43229. doi: 10.1371/journal.pone.0043229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavukcuoglu N.B., Li Q., Pleshko N., Uitto J. Connective tissue mineralization in Abcc6(-/-) mice, a model for Pseudoxanthoma elasticum. Matrix Biol. 2012;31:246–252. doi: 10.1016/j.matbio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terry S.F., Terry P.F., Rauen K.A., Uitto J., Bercovitch L.G. Advocacy groups as research organizations: the PXE International example. Nat Rev Genet. 2007;8:157–164. doi: 10.1038/nrg1991. [DOI] [PubMed] [Google Scholar]
- 36.Belinsky M.G., Kruh G.D. MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheffer G.L., Hu X., Pijnenborg A.C., Wijnholds J., Bergen A.A., Scheper R.J. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- 38.Uitto J., Pulkkinen L., Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment-genome interface? Trends Mol Med. 2001;7:13–17. doi: 10.1016/s1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]
- 39.Luo G., Ducy P., McKee M.D., Pinero G.J., Loyer E., Behringer R.R., Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Q., Li Q., Grand-Pierre A.E., Schurgers L.J., Uitto J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (-/-) mice, a model for pseudoxanthoma elasticum. Cell Cycle. 2011;10:701–707. doi: 10.4161/cc.10.4.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brampton C., Yamaguchi Y., Vanakker O., Van Laer L., Chen L.H., Thakore M., De Paepe A., Pomozi V., Szabo P.T., Martin L., Varadi A., Le Saux O. Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle. 2011;10:1810–1820. doi: 10.4161/cc.10.11.15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorgels T.G., Waarsing J.H., Herfs M., Versteeg D., Schoensiegel F., Sato T., Schlingemann R.O., Ivandic B., Vermeer C., Schurgers L.J., Bergen A.A. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med (Berl) 2011;89:1125–1135. doi: 10.1007/s00109-011-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanakker O.M., Martin L., Schurgers L.J., Quaglino D., Costrop L., Vermeer C., Pasquali-Ronchetti I., Coucke P.J., De Paepe A. Low serum vitamin K in PXE results in defective carboxylation of mineralization inhibitors similar to the GGCX mutations in the PXE-like syndrome. Lab Invest. 2010;90:895–905. doi: 10.1038/labinvest.2010.68. [DOI] [PubMed] [Google Scholar]
- 44.Schurgers L.J., Aebert H., Vermeer C., Bültmann B., Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–3232. doi: 10.1182/blood-2004-04-1277. [DOI] [PubMed] [Google Scholar]
- 45.Rennenberg R.J., van Varik B.J., Schurgers L.J., Hamulyak K., Ten Cate H., Leiner T., Vermeer C., de Leeuw P.W., Kroon A.A. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. 2010;115:5121–5123. doi: 10.1182/blood-2010-01-264598. [DOI] [PubMed] [Google Scholar]
- 46.Weijs B., Blaauw Y., Rennenberg R.J., Schurgers L.J., Timmermans C.C., Pison L., Nieuwlaat R., Hofstra L., Kroon A.A., Wildberger J., Crijns H.J. Patients using vitamin K antagonists show increased levels of coronary calcification: an observational study in low-risk atrial fibrillation patients. Eur Heart J. 2011;32:2555–2562. doi: 10.1093/eurheartj/ehr226. [DOI] [PubMed] [Google Scholar]
- 47.Li Q., Schumacher W., Siegel D., Jablonski D., Uitto J. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br J Dermatol. 2012;166:1107–1111. doi: 10.1111/j.1365-2133.2012.10811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markello T.C., Pak L.K., St Hilaire C., Dorward H., Ziegler S.G., Chen M.Y., Chaganti K., Nussbaum R.L., Boehm M., Gahl W.A. Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutsch F., Nitschke Y., Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]