Figure 2. The brain alters NK cell phenotypes and functions.
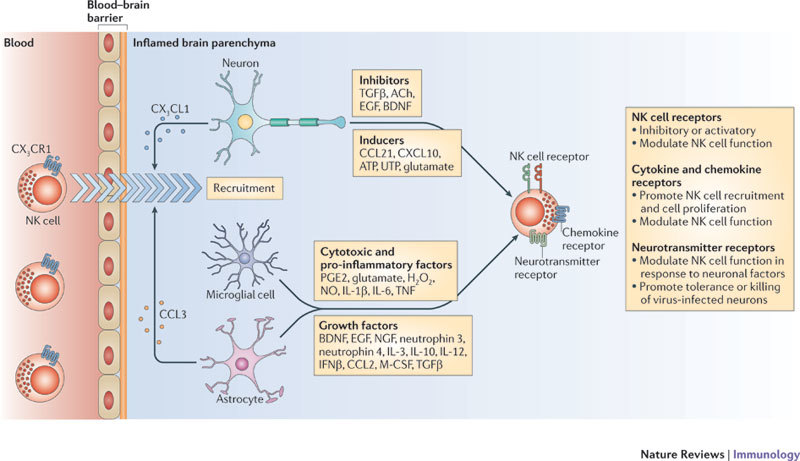
The entry of lymphocytes into the central nervous system (CNS) is normally shielded by the blood–brain barrier, which becomes compromised in several pathological circumstances. Natural killer (NK) cells are recruited to the inflamed brain by CX3C-chemokine ligand 1 (CX3CL1) produced by neurons. Brain-specific cell types and neurotransmitters alter the features of NK cells that migrate from the periphery. An array of cytokines, growth factors and neurotransmitters produced by microglial cells, astrocytes and neurons can influence NK cell activation and proliferation. The chemokine CC-chemokine ligand 3 (CCL3) attracts NK cells to the site of inflammatory foci. After acquiring new features, brain-resident NK cells lose tolerance to microglial cells, suppress pathogenic, myelin-reactive T helper cells and significantly attenuate the intensity of local inflammatory and autoimmune responses. NK cells also kill neurons infected by herpes simplex virus. In addition, mediators derived from neurons, microglial cells and astrocytes and multidirectional cellular interactions may influence NK cell tolerance and function. The combined effects of soluble factors and cellular interactions, as well as the timing of the immune response, may dictate NK cell activity in the CNS. ACh, acetylcholine; BDNF, brain-derived neurotrophic factor; CXCL10, CXC-chemokine ligand 10; EGF, epidermal growth factor; IFNβ, interferon-β; IL, interleukin; M-CSF, macrophage colony-stimulating factor; NGF, nerve growth factor; NO, nitric oxide; PGE2, prostaglandin E2; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor.
