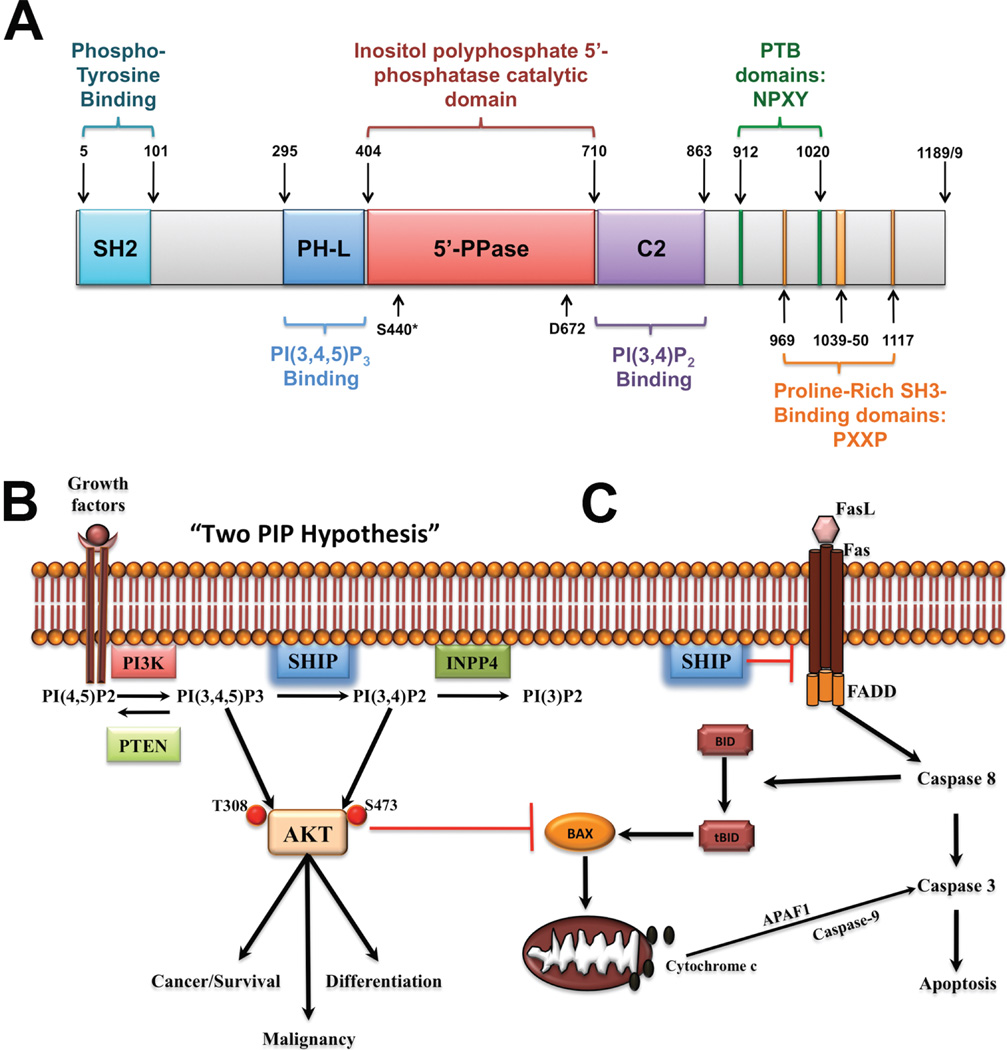
Fig. 1. SHIP1 and its role in cancer and mucosal inflammation.
A. SHIP1 is a 1189 amino acid (1188 aa for isoform b) protein consisting of several different domains, namely the central catalytic domain (5’-PPase) flanked by a PH-like (PH-L) and a C2 domain for binding of the substrate PI(3,4,5)P3 and product PI(3,4)P2 of the enzyme, respectively. The N-terminal region contains a SH2-domain, while the C-terminal “proline-rich” region contains PXXP motifs for interactions with SH3-containing proteins and two NPXY motifs for interaction with Phospho-Tyrosine Binding (PTB) protein. Phosphorylation of Ser440 by PKA has been shown to increase enzymatic activity and the aspartic acid at position 672 (D672) has been identified as a critical catalytic residue. B. The “two PIP hypothesis” suggests that both the substrate, PI(3,4,5)P3, and the product,PI(3,4)P2, of SHIP are necessary to fully activate Akt in order to achieve and maintain the malignant state. As such, selective SHIP1 or pan-SHIP1/2 inhibition can trigger cancer cell death both in vitro and in vivo. C. SHIP may also be involved in regulating Fas trimerization at the cell membrane and would thus serve a protective function against downstream Caspase 8 signaling. Selective SHIP inhibition may therefore have a dual role in triggering apoptosis in cancer cells, via reduced AKT activation and increased Caspase 8 activation. The latter mechanism also suggests that SHIP inhibition may prove useful in reducing inappropriate persistence of autoreactive effector T cells at mucosal surfaces by lifting the inhibition imposed on pathways downstream of Fas.