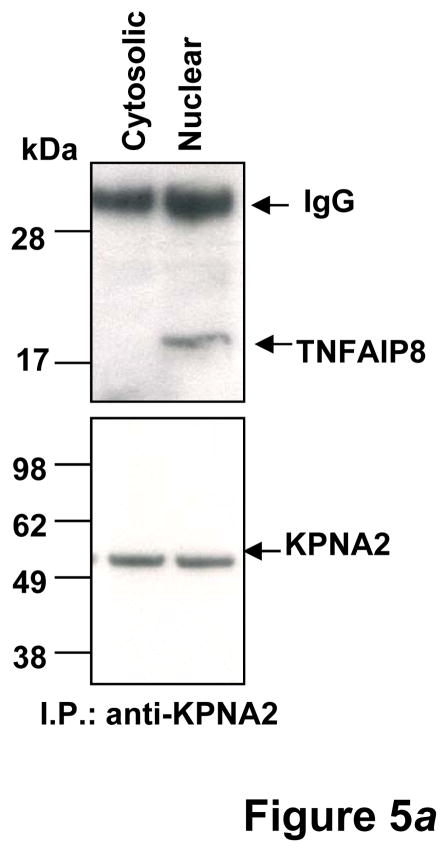
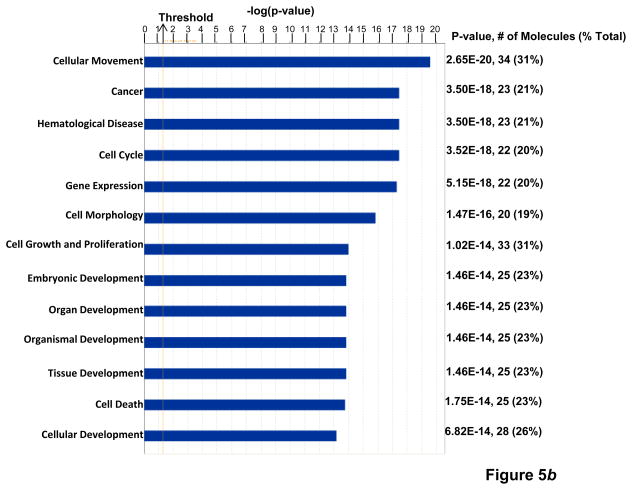
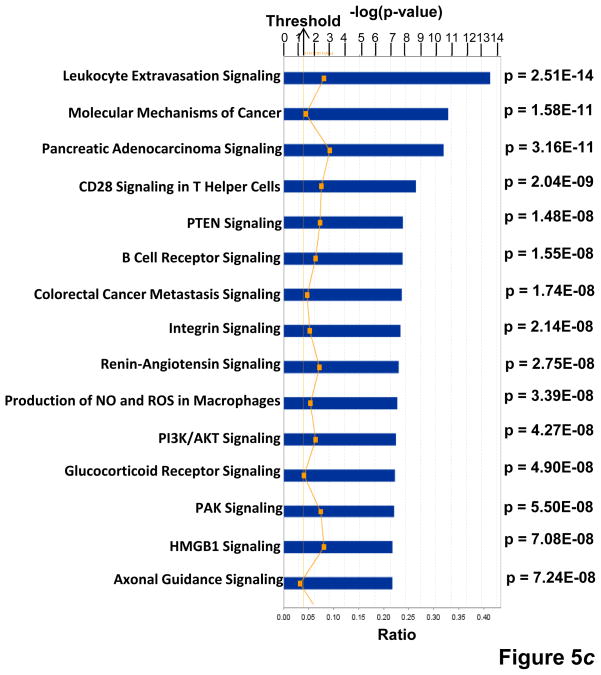
Figure 5.
(a) KPNA2 (importin-α1) is a new binding partner of TNFAIP8. PC-3 cells were fractionated into cytosolic and nuclear fractions and the lysates (1 mg protein) were immunoprecipitated (IP) with anti-Karyopherin α/Rch-1 antibody (3μg), followed by immunoblotting with anti-TNFAIP8 antibody (1:1000 dilution). The blot was reprobed with anti-Importin-α1 antibody (0.2 μg/ml). (b) Top functional categories of TNFAIP8-centric proteins identified in PC-3 cells. TNFAIP8-centric proteins refer to the TNFAIP8 interacting proteins identified in this study by the antibody microarray analysis of TNFAIP8 immune-complexes from PC-3 cells (Supporting Information Table 1) and by the expression analysis in PC-3 cells treated with TNFAIP8 siRNA or scrambled siRNA (Supporting Information Fig. 3). (c) Top canonical pathways in which TNFAIP8 interacting proteins are implicated in PC-3 cells.
 = “p-value” = Chance that the selected molecules fit into the given canonical pathway at random.
= “p-value” = Chance that the selected molecules fit into the given canonical pathway at random.
 = “Ratio” = # of selected molecules in canonical pathway / Total # of molecules in canonical pathway. Threshold = 0.04.
= “Ratio” = # of selected molecules in canonical pathway / Total # of molecules in canonical pathway. Threshold = 0.04.