Fig. 2.
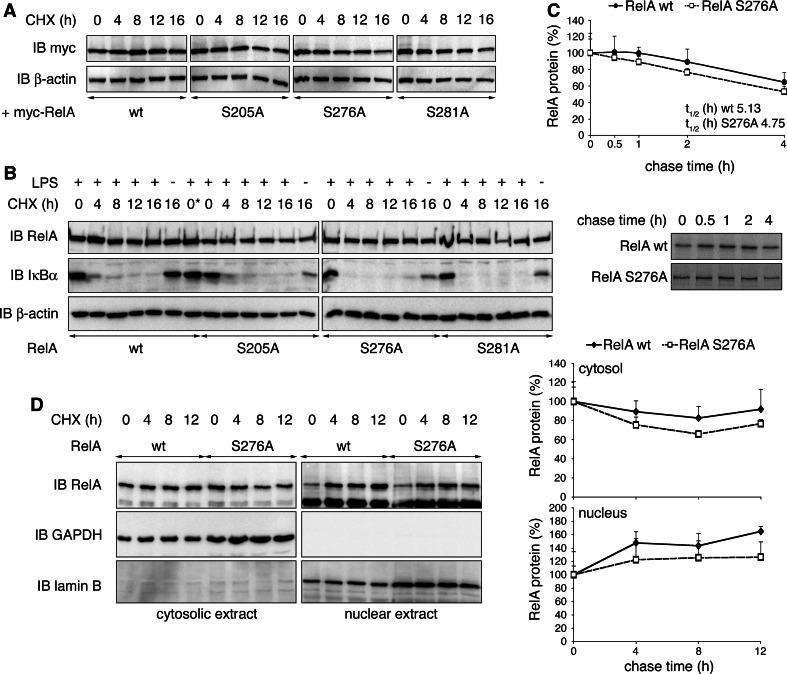
Protein stability of RelA is not influenced by its ubiquitination status. a HEK293T cells were transiently transfected with RelA wt, S205A, S276A or S281A, and treated with cycloheximide (CHX) for indicated time points. Expression of RelA and β-actin was monitored. b RelA−/− 3T3 retrovirally reconstituted with RelA wt or mutants were stimulated for 30 min with LPS and treated with CHX for indicated time points. Immunoblots were probed with RelA-, IκBα- and β-actin-specific antibodies. 0*, w/o addition of CHX, was harvested with 16-h time points. c RelA−/− 3T3 reconstituted with RelA wt or S276A were stimulated with TNF for 30 min in the presence of [35S]-methionine and chased for 0, 0.5, 1, 2 and 4 h in presence of TNF. Immunoprecipitated RelA protein was detected by autoradiography, and RelA protein levels from four experiments were quantified (lower panel = representative autoradiograph). d RelA−/− 3T3 reconstituted with RelA wt or S276A were stimulated for 30 min with LPS and CHX for indicated time points. Cell lysates were separated into cytosolic and nuclear fractions. The presence of RelA was detected with RelA-specific antibody. Separation of cytosol and nuclei was ensured by exclusive detection of GAPDH in cytosolic and lamin B in nuclear fractions. Relative cytosolic and nuclear RelA from two experiments were quantified by normalization to GAPDH and lamin B protein levels, respectively. IB immunoblot