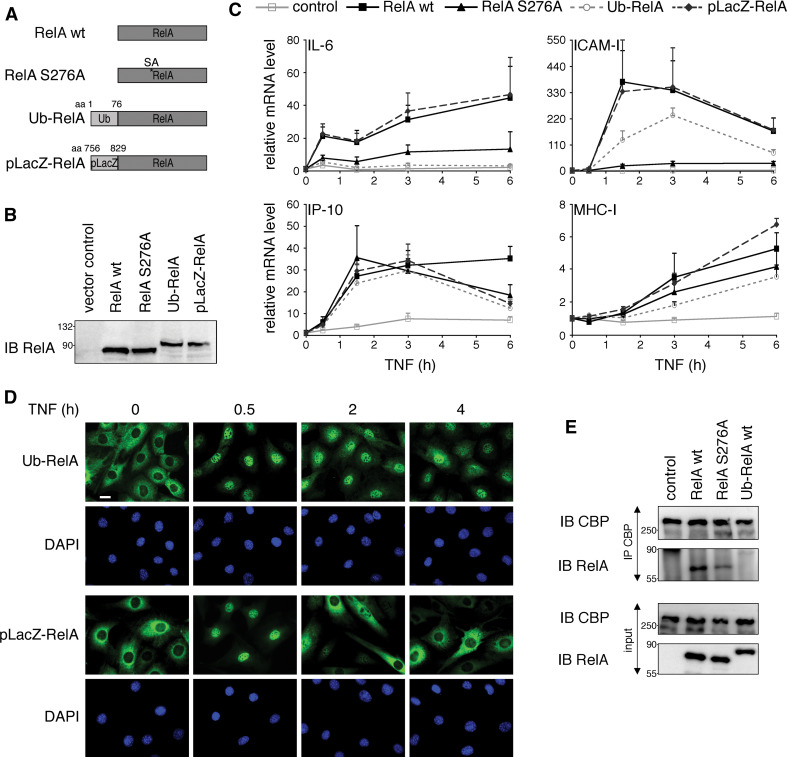
Fig. 7.
Conjugation of monoubiquitin to RelA wt leads to a similar alteration of NF-κB-dependent transcription as RelA S276A mutation. a Schematic representation of RelA constructs used in this study. b Equal expression levels of RelA proteins in 3T3. RelA−/− 3T3 were retrovirally reconstituted with indicated constructs. Expression levels of RelA proteins were monitored by immunoblot with RelA-specific antibody. c Ubiquitin conjugation to RelA wt leads to a similar change in NF-κB transcriptional activity as seen for RelA S276A mutation. RelA−/− 3T3 expressing empty vector (control), RelA wt, RelA S276A, Ub-RelA and pLacZ-RelA were stimulated with TNF for 0.5, 1.5, 3 and 6 h; total RNA was isolated and mRNA levels of IL-6, ICAM-I, IP-10 and MHC-I were analyzed by qPCR. Fold induction of experimental groups was calculated relative to the expression level of unstimulated cells, which was set to 1. Error bars represent mean + SEM of triplicates derived from three independent experiments. d Attachment of ubiquitin but not pLacZ results in prolonged nuclear localization of RelA. RelA−/− 3T3 stably expressing Ub-RelA and pLacZ-RelA were stimulated with TNF for the indicated time points and processed for immunostaining with RelA antibody. Nuclei were stained with DAPI. Size bar 10 μm. e RelA S276A and Ub-RelA bind less efficiently to CBP than RelA wt. Lysates from RelA−/− 3T3 expressing empty vector (control), RelA wt, RelA S276A or Ub-RelA were subjected to immunoprecipitation with CBP antibody. Presence of RelA in precipitates was tested with RelA-specific antibody. IB immunoblot, IP immunoprecipitation