Abstract
IL-10 is a pregnancy compatible cytokine that plays a vital role in maintaining balance of anti-inflammatory and pro-inflammatory milieu at the maternal-fetal interface. Recent evidence now suggests that IL-10 is a potent vascular cytokine that can blunt hypertension and inflammation-mediated vascular dysfunction. Thus, a re-evaluation of IL-10 as a cytokine supporting endovascular interactions and angiogenesis as well as blunting hypoxic-injury and preeclampsia-like features is warranted. In this review, we will highlight these novel functions of IL-10 and propose that its immunomodulatory and vascular functions are mutually inclusive, particularly in the context of normal gestation.
Keywords: IL-10, Angiogenesis, Immune Tolerance, Preeclampsia, Pregnancy
1. Introduction
Although defined as an anti-inflammatory product of T-helper 2 (Th2) cells (Fiorentino, et al., 1989), IL-10 has now been shown to be produced by a wide set of cell types, including both immune and non-immune cells. IL-10 plays a vital role during infection by dampening the immune response to pathogens and consequently controlling the tissue damage in the host. While IL10 expression seems to be regulated at the levels of signal transduction, epigenetics, transcription factor binding, and gene activation (Saraiva and O'Garra 2010), IL-10 is known to function by binding to its cognate receptor IL-10R and activates Janus kinase (JAK) and STAT pathways. This recruits Tyk2 and Jak1 to the receptor complex. The IL-10 receptor is composed of two subunits, IL-10R1 and IL-10R2. Expression of IL-10R is reported on hemopoietic as well as non-hemopoietic cells (Moore et al., 2001). IL-10R1 is constitutively expressed on placental cytotrophoblasts (Szony et al., 1999). IL-10R1 is mainly necessary for the binding of the IL-10 protein while IL-10R2 is specific to initiate a signaling cascade. Jak1 / Tyk2 recruitment induces phosphorylation of the cytoplasmic tail of the receptors and this leads to signal transducer and activator of transcription (STAT)-3 recruitment and phosphorylation which allows homodimerization and entry into the nucleus for transcription of IL-10-regulated genes. Importantly, the STAT3 complex also induces transcription of suppressor of cytokine signaling (SOCS)-3 that triggers a negative feedback loop of IL-10 regulation by blocking subsequent phosphorylation of Jak1 (Thaxton and Sharma, 2010). Although the JAK-STAT pathway is also used by other receptor signaling, the specificity of anti-inflammatory activity of IL-10 is imparted by SOCS-3. IL-10R seems to be refractory to the effects of all members of the SOCS family that performs selective roles in suppressing cytokine signaling from receptors such as gp130 (e.g. IL-6 receptor), the leptin receptor and the granulocyte colony-stimulating factor receptor (Murray, 2006).
IL-10 is now projected as a prominent cytokine that works in a pregnancy compatible manner to sequester over-activation of pro-inflammatory signals at the maternal-fetal interface. Pregnancy complications including preterm birth, fetal demise, and intrauterine growth restriction could primarily be programmed by inflammation or infection in the utero-placental unit. In addition to inflammation and infection, placental vascular deficiency is also a main cause for preeclampsia and in some cases of preterm birth, and this could be due to a direct effect of inflammatory events on placental vasculature. What could be a common protector against inflammatory events and vascular dysfunction? Recent evidence from our laboratory and others clearly suggest that IL-10 functions as a potent anti-inflammatory and vascular cytokine. We discuss its potency against inflammatory and endovascular insults and present evidence that IL-10 supports endovascular cross-talk and inhibits the onset of hypoxia- and serum-induced preeclampsia-like disease in experimental models.
2. Inflammation and pregnancy: Does IL-10 make a difference?
Pregnancy can be separated into three distinct immunological stages: implantation → inflammation, active gestation → anti-inflammation, and parturition → inflammation. Maintenance of this temporal immune programming of pregnancy is critical to successful outcome. The kinetics of IL-10 expression in human placental tissue indicate higher levels during first and second trimesters compared to third trimester of pregnancy, suggesting an immunosuppressive role of IL-10 over the course of human gestation (Hanna, et al., 2000). Different cell types are involved in its production at the maternal-fetal interface. Notably, villous cytotrophoblasts produce IL-10, although it is not clear how IL-10 influence the trophoblast differentiation and invasion. It is possible that the decrease in MMP9 transcription under the influence of IL-10 in villous cytotrophoblasts (Roth, et al., 1996; Roth and Fisher, 1999) may trigger further differentiation to an invasive phenotype. Justifiably, extravillous trophoblasts are intrinsically poor in IL-10 production, thus possibly allowing MMP expression and invasion competency (unpublished data).
Uterine NK (uNK) cells, the major lymphocytes present in the decidua during pregnancy, have the ability to produce and be regulated by IL-10 (Chaouat, et al., 1997; Lidstrom, et al., 2003, Murphy, et al., 2005). Importantly, unlike the primordial immune functions of NK cells, the specialized uNK cells under the influence of pregnancy milieu function to support pregnancy with their unique ability to aid in angiogenesis and placental regulation (Hanna, et al., 2006; Kalkunte et al., 2009a). Decidual macrophages represent another innate immunity cell type that is involved in proper development of the placenta (Lindstrom, et al., 2003). Important insights into the immunological capabilities of uNK cells and decidual macrophages have come from mouse models of pregnancy established in our laboratory and others. In response to inflammatory stimuli such as LPS, a TLR4 agonist, fetal resorption or preterm birth could be observed depending on gestational day (gd) of administration (Murphy, et al., 2005; Robertson et al., 2006; Murphy, et al., 2009). We found that IL-10−/− mice were highly sensitive to low doses of LPS, whereas WT mice required at least a 50-fold higher dose to induce adverse pregnancy outcomes. Importantly, dysregulation of innate immunity was similar in IL-10−/− and WT mice in that uNK cells became cytotoxic, produced TNF-α, and infiltrated the placental zone. It is possible that depending on the type of inflammatory trigger, gestational window of challenge and species, a spectrum of immune cell types may be activated. In this regard, the IL-10−/− mouse model has led to novel concepts in that diverse inflammatory triggers invoke a unique immune response with TNF- α as the detrimental cytokine. For example, in response to TLR9 agonist CpG, negative pregnancy outcomes were induced in IL-10−/− mice by abnormal activation of uterine macrophages, not uNK cells, that produced TNF- α and infiltrated the placental zone (Thaxton, et al., 2009). Taken together, these results demonstrate that in response to inflammatory triggers mimicking bacterial, viral infections, fetal DNA or necrotic tissue, IL-10 is a protective agent.
It is thus conceivable that an anti-inflammatory milieu, perhaps dominated by IL-10, is amplified during pregnancy most likely as a mechanism of tolerance toward the fetal allograft. There have been several studies that associate IL-10 deficiency to adverse pregnancy outcomes such as recurrent spontaneous abortion (RSA), preterm birth, and pre-eclampsia (Hill, et al., 1995; Hennessy, et al., 1999; Raghupathy, et al., 1999; Rezaei and Dabbagh, 2002; Plevyak et al., 2002; Makhseed, et al., 2003). The mechanisms that may lead to poor IL-10 production at the maternal–fetal interface are not well understood. However, polymorphisms in the IL-10 gene promoter have been associated with dysregulated IL-10 production and several diseases. Recent studies have identified five SNPs at −3575, −2849, −1082, −819, and −592 positions in the human IL-10 gene promoter (Makris et al., 2006; Zammiti, et al., 2006; Kamali-Sarvestani, et al., 2006). Similarly, the molecular effects of these SNPs in the IL-10 gene promoter in pregnant women remain to be elucidated in the context of pregnancy complications. However, recent studies demonstrate cell type–specific effects of the genotypic changes in the IL-10 gene promoter. Further, these studies showed that the responses may be influenced by bacterial infections or other inflammatory conditions to suppress IL-10 production in human trophoblasts (Sharma et al, 2010a). It is important to note that various cell types can produce IL-10 (Saraiva and O'Garra 2010), and consequently, it is expected that expression of IL-10 in different cell types is regulated uniquely in a cell type-specific manner. Further investigations are warranted to answer these important issues.
3. Evidence for vascular IL-10
It is prudent to say now that the functions of IL-10 should be expanded to include not only the immunomodulatory activities but also the protective role in hypertension- or inflammation-induced vascular dysfunction. Recent studies from our lab showed that exposure to environmental toxicants such as polychlorinated biphenyls (PCBs) result in preterm birth, intrauterine growth restriction and two-fold increase in amniotic fluid only in IL-10−/− pregnant mice (Tewari, et al., 2009). At the maternal-placental interface, impaired spiral artery remodeling and placental angiogenesis were observed. Mechanistically, these perturbations were associated with reduced placental protein levels of aquaporin-1 (Tewari, et al., 2009) and perturbed VEGF receptor-Dll4-Notch signaling pathway (unpublished observation) under IL-10 deficiency with minimal effects in wild type counterparts. This suggested a protective role of IL-10 in amniotic fluid regulation, preterm birth and angiogenesis. Indeed when recombinant IL-10 was co-injected along with PCBs, there was complete rescue of preterm birth and IUGR, amniotic fluid regulation, and aquapoin-1 expression to normal pregnancy levels. We have developed a novel in vitro model of endovascular activity that recapitulates the interaction between first trimester extravillous trophoblast (EVT) lacking IL-10 production and endothelial cells and closely mimics decidual spiral artery remodeling in response to serum from normal pregnancy (Kalkunte, et al., 2008). Using this model, PCBs disrupted the endovascular activity which was rescued by exogenous IL-10 (Tewari et al., 2009). Likewise, IL-10 has been shown to protect against LPS- or angiotensin II-induced vascular dysfunction (Gunnett, et al., 1999; Didion, et al., 2009). In an experimental model of pregnancy, IL-10 has also been shown to attenuate fetal growth restriction and demise (Rivera, et al., 1998).
4. IL-10 and preeclampsia
Preeclampsia (PE) is a late pregnancy malady that occurs in 5–10% of pregnancies worldwide. PE is a systemic disorder resulting from poor placentation. Although the pathogenesis of PE remains poorly understood, improper trophoblast invasion and poor spiral artery remodeling resulting in placental ischemia/hypoxia are the major pre-clinical events at the maternal-fetal interface (Brosens, et al., 1977; Meekins et al., 1994; Redman and Sargent 2009; Kalkunte, et al., 2009b). As a consequence, placenta-derived flux of inflammatory molecules and anti-angiogenic factors are observed in maternal systemic circulation resulting in endothelial dysfunction and symptoms of hypertension, proteinuria and kidney pathology (Levine, et al., 2004, Parikh and Karumanchi, 2008). The central role played by placenta in the onset of this disorder is apparent as the symptoms resolve with delivery. In this context recent studies from our lab have suggested that reduced production of IL-10 may contribute to poor placentation and induction of vasoactive anti-angiogenic factors. Curiously, evaluation of placental tissue and serum samples from PE has suggested reduced IL-10 production (Wilczyński, et al., 2002; Hennessy, et al., 1999). There may also be a genetic link to pre-eclampsia, and analysis of SNPs in the IL-10 gene promoter is likely to provide insights into the nature of this disease. Interestingly, one genotype, −2849AA, is thought to be associated with a threefold reduced risk toward acquisition of pre-eclampsia (De Groot, et al., 2004).
Recent data from our group describing a serum-based pregnancy-specific mouse model of preeclampsia and an in vitro predictive assay are intriguing. The in vivo model using IL-10−/− mice closely mirrors the human condition and is pregnancy specific (Kalkunte, et al., 2010). We showed that a single administration of human preeclampsia serum in pregnant IL-10−/− mice induced the full spectrum of preeclampsia-like symptoms including IUGR, hypertension, proteinuria and kidney pathology. The same serum sample(s) induced a partial preeclampsia phenotype in wild type mice. Mechanistically, in the absence of IL-10, these serum samples impaired spiral artery transformation, caused hypoxic injury in uteroplacental tissue, and triggered excess production of soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng). We have recently undertaken a large scale proteomic analysis of preeclampsia serum samples and have observed dysregulation of some novel proteins, which when added to preeclampsia serum reverse its disease causing effects (Sharma, et al., 2009a; 2009b)
Although hypoxia (pathologic O2 levels) has been associated with the onset of preeclampsia, no in vivo evidence exists to support this notion and the role of IL-10 in blunting hypoxia effects. It is possible that extent and severity of placental hypoxia may define the placental release of cytotoxic and anti-angiogenic factors into circulation leading to the onset of systemic preeclampsia symptoms (Sharma, et al., 2010b). In this context, in a recent study from our lab, we sought to define the pathologic levels of hypoxic perturbation and to establish its “causal” link to preeclampsia in vivo. In this study, exposure of pregnant wild type and IL-10−/− mice to 9.5% O2 resulted in graded placental injury and systemic symptoms of PE (Lai, et al., 2009). These features were consequence of marked elevation of sFlt-1, sEng and placental apoptosis mediated through the p53 signaling pathway. Importantly, recombinant IL-10 reversed hypoxia-induced features in pregnant IL-10−/− mice confirming the protective role of IL-10 in preeclampsia (Lai, et al., 2010). It is thus possible that severity of preeclampsia pathology is associated with the extent of low oxygen levels and intrinsic IL-10 deficiency.
These results strongly suggest a moderating role of IL-10 in placental angiogenesis, hypoxia/ischemia and preeclampsia. This notion is further supported by a recent observation that exogenous IL-10 can normalize blood pressure and endothelial function in pregnancy-induced hypertensive rats. In these studies, the beneficial effects of IL-10 in pregnant DOCA/saline-treated (PDS) rats were associated with decreased plasma levels of endothelin-1, decreased levels of circulating and placental IFN-γ, as well as decreased aortic and placental expression of PECAM although the effect on placental angiogenesis and spiral artery remodeling effects are not clear (Tinsley, et al., 2010).
5. Conclusions
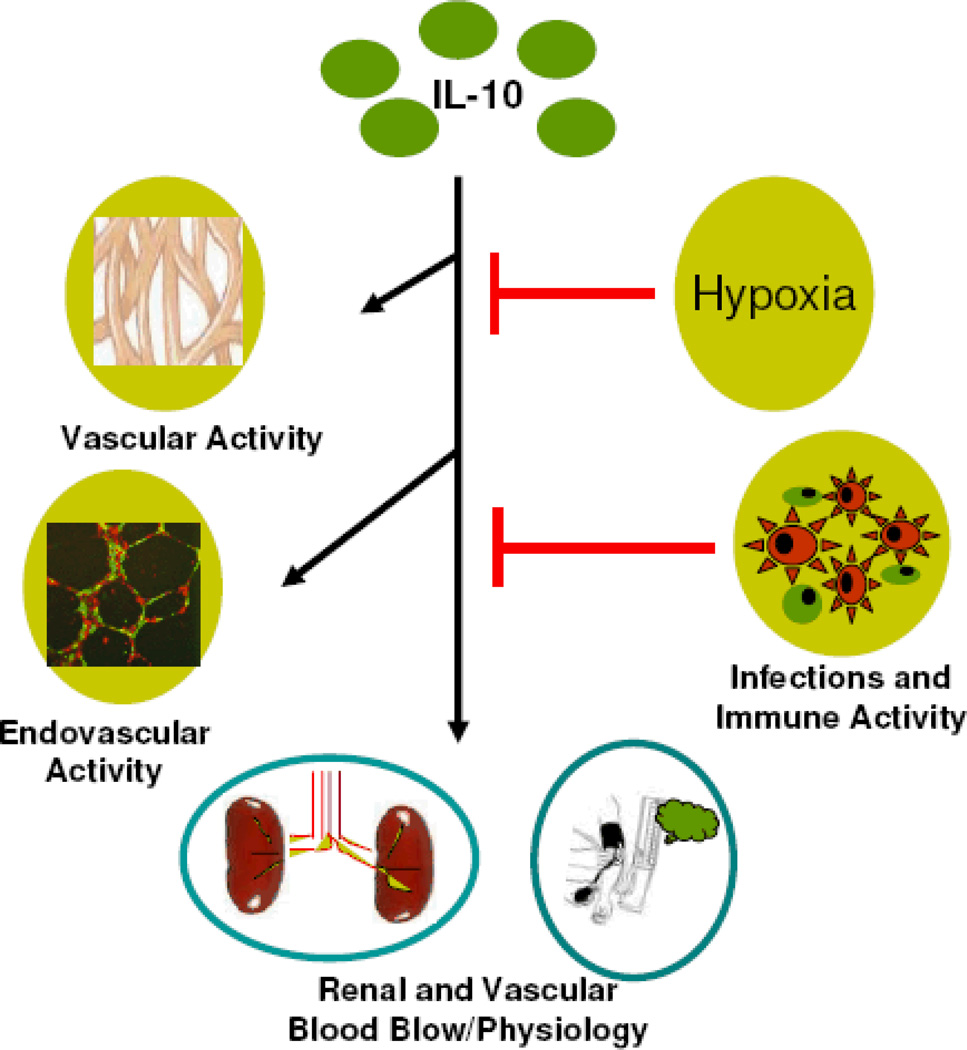
In addition to the protective role of IL-10 in inflammation, we propose that IL-10 functions as a vital bridge that links immunity, placental angiogenesis, and hypoxia at the maternal-fetal interface (Fig 1). Our work in IL-10−/− mice for the first time provides important clues to the pathogenesis of preeclampsia. These observations suggest the possibility of IL-10-based therapy for adverse pregnancy outcomes, particularly when personalized medicine becomes a reality for enigmatic maladies.
Fig. 1. Immune-Vascular Interactions of IL-10: Implications in Preeclampsia.
Interleukin (IL)-10 modulates vascular activity and endovascular interactions at the maternal-fetal interface in addition to its known anti-inflammatory and immune-regulatory functions. This ensures normal renal physiology and blood pressure during pregnancy. Hypoxia is known to down regulate IL-10 expression (Bowen et al., 2005). In addition, infection and ensuing inflammation may adversely influence cytokine balance and normal physiological functions of IL-10 predisposing onset of preeclampsia.
Acknowledgements
This work was supported in part by a grant from NIH P20RR018728 and by the Rhode Island Research Alliance Collaborative Research Award 2009-28.
References
- Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J. Soc. Gynecol. Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br. J. Obstet. Gynaecol. 1977;84(9):656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Tranchot Diallo J, Volumenie JL, Menu E, Gras G, Delage G, Mognetti B. Immune suppression and Th1/Th2 balance in pregnancy revisited: a (very) personal tribute to Tom Wegmann. Am. J. Reprod. Immunol. 1997;37:427–434. doi: 10.1111/j.1600-0897.1997.tb00255.x. [DOI] [PubMed] [Google Scholar]
- De Groot CJ, Jansen MW, Bertina RM, Schonkeren JJ, Helmerhorst FM, Huizinga TW. Interleukin 10-2849AA genotype protects against pre-eclampsia. Genes. Immun. 2004;5:313–314. doi: 10.1038/sj.gene.6364092. [DOI] [PubMed] [Google Scholar]
- Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54(3):619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnett CA, Berg DJ, Faraci FM, Feuerstein G. Vascular effects of lipopolysaccharide are enhanced in interleukin-10-deficient mice. Stroke. 1999;30(10):2191–2195. doi: 10.1161/01.str.30.10.2191. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J. Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273:1933–1936. [PubMed] [Google Scholar]
- Kalkunte S, Boij R, Norris W, Friedman J, Lai Z, Kurtis J, Lim KH, Padbury JF, Matthiesen L, Sharma S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am. J. Pathol. 2010 doi: 10.2353/ajpath.2010.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkunte S, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. VEGF C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J. Immunol. 2009a;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkunte S, Lai Z, Norris WE, Pietras LA, Tewari N, Boij R, Neubeck S, Markert UR, Sharma S. Novel approaches for mechanistic understanding and predicting preeclampsia. J Reprod Immunol. 2009b;83:134–138. doi: 10.1016/j.jri.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkunte S, Lai Z, Tewari N, Chichester C, Romero R, Padbury J, Sharma S. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta. 2008;29:871–878. doi: 10.1016/j.placenta.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali-Sarvestani E, Kiany S, Gharesi-Fard B, Robati M. Association study of IL-10 and IFN-gamma gene polymorphisms in Iranian women with preeclampsia. J. Reprod. Immunol. 2006;72:118–126. doi: 10.1016/j.jri.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lai Z, Kalkunte S, Sharma S. Pregnancy-specific effects of hypoxia: a mouse model for preeclampsia. Am. J. Reprod. Immunol. 2009;61:398. [Google Scholar]
- Lai Z, Kalkunte S, Sharma S. A critical role of IL-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.110.163329. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am. J. Reprod. Immunol. 2003;50:444–452. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- Makhseed M, Raghupathy R, El-Shazly S, Azizieh F, Al-Harmi JA, Al-Azemi MM. Pro-inflammatory maternal cytokine profile in preterm delivery. Am. J. Reprod. Immunol. 2003;49:308–318. doi: 10.1034/j.1600-0897.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency ofinterleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta. 2006;27:445–451. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- Moore KW, De Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J. Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Hanna NN, Fast LD, Shaw S, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am. J. Obstet. Gynecol. 2009;200:308, e1–e9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat. Med. 2008;14:855–862. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- Plevyak M, Hanna N, Mayer S, Murphy S, Pinar H, Fast L, Ekerfelt C, Ernerudh J, Berg G, Matthiesen L, Sharma S. Deficiency of decidual IL-10 in first trimester missed abortion: a lack of correlation with the decidual immune cell profile. Am J Reprod Immunol. 2002;47:242–250. doi: 10.1034/j.1600-0897.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196:122–130. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30:S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Rezaei A, Dabbagh A. T-helper (1) cytokines increase during early pregnancy in women with a history of recurrent spontaneous abortion. Med. Sci. Monit. 2002;8:CR607–CR610. [PubMed] [Google Scholar]
- Rivera DL, Olister SM, Liu X, Thompson JH, Zhang XJ, Pennline K, Azuero R, Clark DA, Miller MJ. Interleukin-10 attenuates experimental fetal growth restriction and demise. FASEB J. 1998;12(2):189–197. doi: 10.1096/fasebj.12.2.189. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J. Immunol. 2006;177:4888–96. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth I, Fisher SJ. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev. Biol. 1999;205:194–204. doi: 10.1006/dbio.1998.9122. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Sharma S, Stabila J, Pietras L, Singh AR, McGonnigal B, Ernerudh J, Matthiesen L, Padbury JF. Haplotype-dependent differential activation of the human IL-10 gene promoter in macrophages and trophoblasts: Implications for placental IL-10 deficiency and pregnancy complications. Am. J. Reprod. Immunol. 2010a;64:179–187. doi: 10.1111/j.1600-0897.2010.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Norris WE, Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J Reprod Immunol. 2010b;85:112–116. doi: 10.1016/j.jri.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Neubeck S, Norris W, Markert UR, Kalkunte S. Serum-based diagnosis and mouse models for preeclampsia. Z. Geburtsh. Neonatol. 2009a;213:71. [Google Scholar]
- Sharma S, Kalkunte S, Markert U. Compositions, formulations and methods of treating preeclampsia-type disorders of preeclampsia. 2009b US 61/207,026 and WO/2010/091253. [Google Scholar]
- Szony BJ, Bata-Csörgo Z, Bártfai G, Kemény L, Dobozy A, Kovács L. Interleukin-10 receptors are expressed by basement membrane anchored, alpha(6) integrin(+) cytotrophoblast cells in early human placenta. Mol. Hum. Reprod. 1999;5:1059–1065. doi: 10.1093/molehr/5.11.1059. [DOI] [PubMed] [Google Scholar]
- Tewari N, Kalkunte S, Murray DW, Sharma S. The water channel aquaporin 1 is a novel molecular target of polychlorinated biphenyls for in utero anomalies. J. Biol. Chem. 2009;284:15224–15232. doi: 10.1074/jbc.M808892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J. Immunol. 2009;183:1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R713–R719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- Wilczyński JR, Tchórzewski H, Głowacka E, Banasik M, Lewkowicz P, Szpakowski M, Zeman K, Wilczyński J. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators. Inflamm. 2002;11:105–111. doi: 10.1080/09629350220131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammiti W, Mtiraoui N, Cochery-Nouvellon E, Mahjoub T, Almawi WY, Gris JC. Association of-592C/A,-819C/T and -1082A/G interleukin-10 promoter polymorphisms with idiopathic recurrent spontaneous abortion. Mol. Hum. Reprod. 2006;12:771–776. doi: 10.1093/molehr/gal084. [DOI] [PubMed] [Google Scholar]