Abstract
Purpose
Volume expansion is a common therapeutic intervention in septic shock, although patient response to the intervention is difficult to predict. Central venous pressure (CVP) and shock index have been used independently to guide volume expansion, although their use is questionable. We hypothesize that a combination of these measurements will be useful.
Methods
In a prospective, observational study, patients with early septic shock received 10-mL/kg volume expansion at their treating physician’s discretion after brief initial resuscitation in the emergency department. Central venous pressure and shock index were measured before volume expansion interventions. Cardiac index was measured immediately before and after the volume expansion using transthoracic echocardiography. Hemodynamic response was defined as an increase in a cardiac index of 15% or greater.
Results
Thirty-four volume expansions were observed in 25 patients. A CVP of 8 mm Hg or greater and a shock index of 1 beat min−1 mm Hg−1 or less individually had a good negative predictive value (83% and 88%, respectively). Of 34 volume expansions, the combination of both a high CVP and a low shock index was extremely unlikely to elicit hemodynamic response (negative predictive value, 93%; P = .02).
Conclusions
Volume expansion in patients with early septic shock with a CVP of 8 mm Hg or greater and a shock index of 1 beat min−1 mm Hg−1 or less is unlikely to lead to an increase in cardiac index.
Keywords: Sepsis, Fluid, Shock index, Central venous pressure
1. Introduction
Volume expansion through intravenous administration of crystalloid or colloid is frequently performed in patients with septic shock and is often one of the first therapies performed. The goal of volume expansion is to increase cardiac index (CI), thereby improving organ perfusion. Volume expansion carries known risks, however, and has been associated with increased mortality and morbidity [1–3]. With the widespread adoption of early goal–directed therapy (EGDT) by Rivers et al [4] and Dellinger et al [5], central venous pressure (CVP) is measured in many patients with septic shock and is commonly used to justify volume expansion. Despite clinical enthusiasm for measuring CVP, several groups have observed that CVP alone fails to reliably predict a hemodynamic response (increase in CI) to volume expansion [6–8]. Furthermore, some patients with high CVP will respond to volume expansion. Strict adherence to the EGDT algorithm may fail to provide volume expansion to patients who could benefit from it.
Some investigators suggest that “dynamic” measures, which vary with respiration or with passive leg raise, are superior to “static” measures at identifying patients who would benefit from volume expansion [9–14]. Clinicians hoping to enhance EGDT practice propose replacing static measures with newer dynamic measures in their sepsis management protocols [14]. However, many of these dynamic measures are not readily available at the bedside. The passive leg-raise technique, for example, requires personnel time and a device to measure CI [15]. Despite enthusiasm for goal-directed protocols, there are limitations inherent to using a single hemodynamic value (static or dynamic) to characterize a patient. An assessment or treatment protocol based on a single hemodynamic value may be less informative than the one incorporating multiple values.
It is not known whether the measurement of an additional hemodynamic parameter could improve the predictive use of CVP. The shock index (SI), the ratio of heart rate (beat/min) to systolic blood pressure (mm Hg), is a simple hemodynamic parameter easily obtained at the bedside and has been used to identify critically ill hypovolemic patients in the emergency department [16]. Shock index previously has been demonstrated to have an inverse relationship to left ventricular stroke volume and CI in septic shock [16,17]. We hypothesized that a composite measurement of CVP and SI would better predict a hemodynamic response (an increase in CI) to volume expansion when compared with CVP alone.
2. Materials and methods
2.1. Study design
This prospective, observational study was conducted between January 2010 and August 2011 in the 24-bed shock/trauma intensive care unit (ICU) and the 12-bed respiratory ICU at the Intermountain Medical Center, an academic, tertiary-care hospital in Murray, Utah. The protocol was approved by the Intermountain Healthcare institutional review board, and all patients or their legally authorized representatives provided written, informed consent.
2.2. Patients
We screened patients admitted to the ICU with septic shock defined by standard criteria [18]. The patients were enrolled within 24 hours of the initial resuscitation of septic shock. We included patients who were at least 14 years of age with suspected infection and 2 or more systemic inflammatory response syndrome criteria (white blood cell count <4000/mm3 or >12 000/mm3 or differential with >10% immature forms, heart rate >90 beats/min, respiratory rate >20 breaths/min or Paco2 <32 mm Hg, temperature <36°C or >38°C), evidence of refractory hypotension (a systolic blood pressure <90 mm Hg despite intravenous volume expansion of at least 20 mL/kg), and in whom the attending physician intended to perform volume expansion. When the treating physicians decided that volume expansion was clinically indicated, 10 mL/kg crystalloid or equivalent colloid was infused intravenously over a period of less than 20 minutes. We excluded patients with known pregnancy, irregular rhythm (atrial fibrillation or frequent premature ventricular contractions), contraindication to central venous catheterization or arterial catheterization, or in whom the attending physician deemed aggressive care unsuitable. The reason for exclusion of irregular ventricular rhythm was the potential for erroneous echocardiographic assessment of CI in these patients because stroke volume may change with each beat. Patients were observed for a period of 6 hours after study enrollment. If a second volume expansion was administered during that period, it was also included in the analysis. A volume expansion was excluded if there was a change in vasopressor administration simultaneous with volume expansion. All patients were managed in the emergency department and after admission to the ICU according to Surviving Sepsis Campaign Guidelines 2008 [5] as included in the “sepsis bundle” used at Intermountain Medical Center.
2.3. Assessing CI
We used transthoracic echocardiography (TTE) to assess CI, according to standard technique [19,20]. Images were obtained using a Philips CX50 ultrasound system (Philips Medical Systems, Bothell, Wash). We performed serial limited TTE examinations immediately before and immediately after each volume expansion. The CI was calculated using velocity-time integration and left ventricular outflow tract diameter and was normalized to body surface area, averaged over 3 consecutive ventricular contractions obtained at end-expiration. All cardiac echocardiograms were performed by a single physician (M.L.). All echocardiograms were interpreted by M.L., with a second, independent reviewer (E.H.) overreading the echocardiograms. The reviewing physician was blinded to the clinical outcome. The interpreting and reviewing physicians are testamurs of the National Board of Echocardiography Examination of Special Competence in Adult Echocardiography and regularly perform and interpret echocardiograms in the clinical setting. The treating physicians were blinded to the results of the limited TTE until after the completion of the study.
2.4. Clinical data
Immediately before volume expansion, CVP, heart rate, and systolic blood pressure were obtained. All CVP measurements were obtained at end-expiration with the patient in a supine position and with the pressure transducer positioned at the level of the right atrium. Shock index was calculated as heart rate/systolic blood pressure [17]. Systolic blood pressure was obtained from a radial arterial catheter. In addition, we obtained vital signs, mechanical ventilation parameters, types and doses of vasoactive medications being infused, total volume of crystalloid solution infused since arrival in the emergency department, and total urine output in the period from arrival in the emergency department to the time the volume expansion was performed.
2.5. Primary analysis
We assessed the role of CVP and SI in predicting response to volume expansion. Our primary outcome was hemodynamic response, defined as an increase in a CI of 15% or greater after volume expansion.
2.6. Statistical methods
Receiver operating characteristic (ROC) curves were calculated for CVP and SI. The composite of CVP and SI was calculated by logistic regression and was evaluated with area under the ROC curve (AUC), with goodness of fit evaluated by the Hosmer-Lemeshow technique (c statistic); comparisons of AUC were made using the technique of DeLong et al [21]. Group comparisons used Fisher exact test, Student t test, or Wilcoxon rank sum, where appropriate. All analyses were performed using Stata v12 (StataCorp, College Station, Tex).
3. Results
3.1. Clinical and demographic data
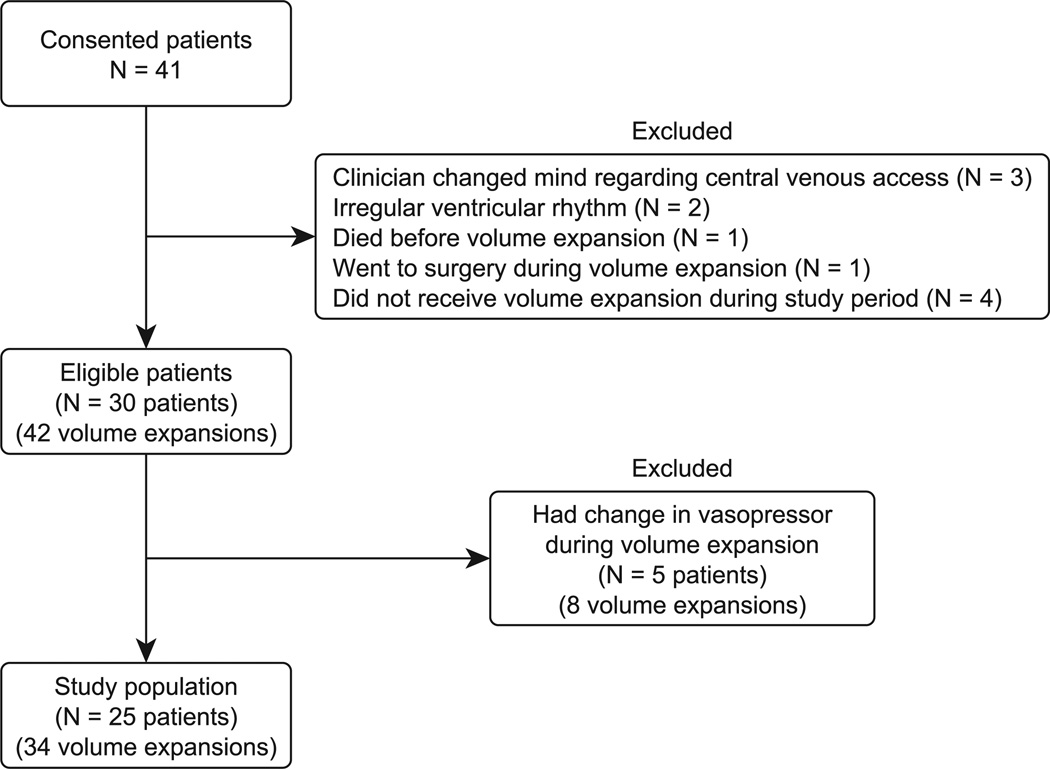
We studied 25 patients, with a total of 34 volume expansions. Excluded patients are depicted in Fig. 1. Eight volume expansions (5 patients) were excluded because of a dose change of vasoactive medications during the volume expansion. Demographic and clinical information is displayed in Table 1. Eleven patients (19 volume expansions) were undergoing mechanical ventilation at the time of volume expansion, and 19 patients (26 volume expansions) were receiving vasoactive medications. Only 2 patients were chemically paralyzed at the time of volume expansion. No patient had active exacerbation of chronic obstructive pulmonary disease or asthma. No patient had concomitant receipt of a β-agonist or β-blocker during the fluid challenge, although 7 patients received a scheduled bronchodilator at some point during the day of the study. One patient received a β-blocker during the day of the study. Patients received a median of 4.6 L of crystalloid before enrollment. Nineteen patients were initially resuscitated in our emergency department before enrollment, with a median duration of 2 hours and a median amount of crystalloid equal to 3 L. Study patients all were enrolled within 8 hours of meeting the criteria for septic shock (median, 3.1 hours). Seven patients were resuscitated at a referring facility before transport to our ICU (median time before transfer, 6.25 hours), and 3 were inpatients before admission to the ICU (the median duration of resuscitation before enrollment was 12 hours).
Fig. 1.
Patient enrollment description.
Table 1.
Baseline demographics (median, interquartile ranges)
| Responders (10) | Nonresponders (24) | P | |
|---|---|---|---|
| Age (y) | 59 (46–72) | 59 (54–77) | .52 |
| Percent female | 60 | 71 | .69 |
| APACHE-2 score | 19 (15–21) | 19 (15–28) | .81 |
| Percent on vasopressor | 70 | 79 | .67 |
| Percent on ventilator | 40 | 50 | .71 |
| Fluid administered before enrollment (L) | 4.0 (3.0–5.0) | 5.5 (3.1–6.0) | .34 |
| Cardiac index (L min−1 m−2) | 3.83 (2.57–4.46) | 2.83 (2.05–3.25) | .21 |
| Heart rate (beats/min) | 111 (95–125) | 94 (81–113) | .02 |
| Systolic blood pressure (mm Hg) | 90 (88–107) | 94 (85–106) | .99 |
| Mean arterial blood pressure (mm Hg) | 65 (61–77) | 65 (62–74) | .93 |
| Temperature (ºC) | 36.3 (35.4–37.0) | 36.6 (35.9–37.9) | .38 |
| Respiratory rate (breaths/min) | 26 (17–30) | 21 (18–32) | .78 |
| Central venous pressure (mm Hg) | 7 (5–12) | 11 (9–16) | .04 |
| Shock index (beat min−1 mm Hg−1) | 1.12 (1.02–1.35) | 0.95 (0.84–1.15) | .07 |
3.2. Hemodynamic response
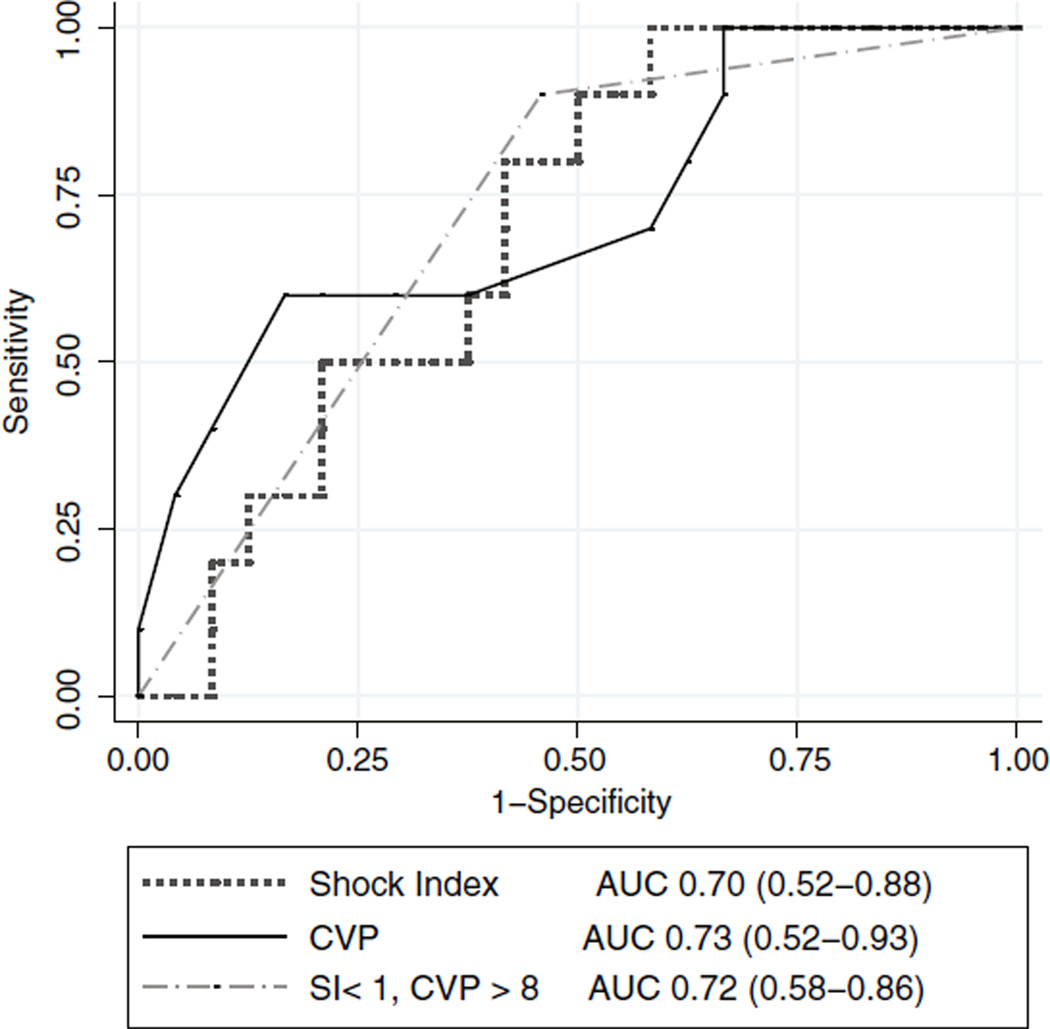
Ten of 34 volume expansions resulted in an increase in a CI of 15% or greater. The median change in CI for all volume expansions was +11.0%. There was a significant difference in CVP between responders and nonresponders (Table 1; Table E1). The CVP had an area under the AUC of 0.73, whereas SI had an AUC of 0.70, which indicate moderate predictive utility (Fig. 2). Sensitivity analysis revealed that a CVP threshold of 8 mm Hg yielded a negative predictive value (NPV) of 83% (95% confidence interval, 62%–95%) and a positive predictive value (PPV) of 60% (27%–86%; Table 2). Similarly, an SI threshold of 1 beat min−1 mm Hg−1 yielded an NPV of 88% (60%–98%) and a PPV of 44% (22%–69%). Among patients with both a CVP of 8 mm Hg or greater and an SI of 1 beat min−1 mmHg−1 or less (n = 13), hemodynamic improvement was unlikely to occur with volume expansion (NPV, 93%; 71%–100%; P = .02). The composite of high CVP and low SI demonstrated an AUC of 0.72 (Fig. 2). The AUC did not differ significantly for CVP, SI, or the combination. The presence of both CVP less than 8 mm Hg and SI greater than 1 beat min−1 mm Hg−1 was less useful for predicting response to volume expansion (PPV, 63%; 26%–90%; P = .03). If the analysis was restricted to only the first volume expansion for each patient (25 volume expansions), the presence of a CVP of 8 mm Hg or greater and an SI of 1 beat min−1 mm Hg−1 or less still yielded a high NPV of 91% (66%–100%, P = .03).
Fig. 2.
Receiver-operating characteristic curves predicting hemodynamic response to volume expansion. Depicted are curves comparing CVP, SI, and the composite of a CVP of 8 mm Hg or greater and an SI of 1 beat min−1 mm Hg−1 or less. The AUCs are calculated, with 95% confidence intervals in parentheses.
Table 2.
Distribution of volume expansion by CVP and SI, for all volume expansions
| All patients | CVP <8 mm Hg |
CVP ≥ 8 mm Hg |
SI > 1 beat min−1 mm Hg−1 |
SI ≤ 1 beat min−1 mm Hg−1 |
CVP <8 mm Hg or SI >1 beat min−1 mm Hg−1 |
CVP ≥8 mm Hg and SI ≤1 beat min−1 mm Hg−1 |
|---|---|---|---|---|---|---|
| Nonresponders | 4 | 20 | 10 | 14 | 11 | 13 |
| Responders | 6 | 4 | 8 | 2 | 9 | 1 |
| PPV (95% CI) | 60% (27% –86%) | 44% (22% –69%) | 45% (30% –50%) | |||
| NPV (95% CI) | 83% (62% –95%) | 88% (60% –98%) | 93% (71% –100%) | |||
| P | .03 | .06 | .02 | |||
Comparison (P value) using Fisher exact test.
CVP=central venous pressure; SI=shock index; PPV=positive predictive value; NPV=negative predictive value.
4. Discussion
This prospective evaluation of predictors of hemodynamic responsive to volume expansion suggests that patients in early septic shock (after early emergency department resuscitation) with an elevated CVP and a normal SI do not exhibit a positive hemodynamic response to volume expansion. This composite measurement, among those with high CVP and relatively low SI, performs better than either CVP or SI alone in predicting a negative response to volume expansion. Because the state of elevated CVP and low SI is the most informative of the combinations studied, AUC is a relatively less informative measure of comparative or overall utility. We emphasize that presence of a low CVP or a high SI or both does not reliably predict a successful volume expansion in septic shock, and their composite is not any better. On the basis of our data, CVP and SI are primarily useful for identifying patients who will not benefit from volume expansion.
The future of critical care management likely will incorporate multiple measurements and sequential hypothesis testing. More expensive or more difficult methods of assessment may be reserved for cases where initial assessment is uncertain. A multimodal assessment of fluid status and response to volume expansion may be a better use of ICU personnel and resources. One might use CVP and SI, which are easily and commonly obtained, as an important screening test before performing a passive leg raise maneuver or before using a more expensive confirmatory test.
We highlight from our findings that only 32% of patients remain volume responsive after a brief stay in the contemporary emergency department. This number is within the range of results (30%–72%) previously reported by other investigators and is a reminder that many critically ill patients are not volume responsive [9,22,23]. In the aftermath of the 2001 publication of Dellinger et al [5] and McIntyre et al [24], early and aggressive volume expansion is becoming standard practice. Despite very brief emergency department stays (median time, 2 hours), patients in this study had received considerable volume expansion (median, 3 L) before admission to the ICU. We suspect that this aggressive fluid resuscitation is a result of wide dissemination of the Surviving Sepsis Campaign bundle at our hospital [5]. We also believe there may be some selection bias involved because measurement of CVP requires placement of a central venous catheter. By the time the catheter has been placed, the patient may have received substantial volume expansion. Because the patients in this study all had received volume expansion before enrollment, this study is most applicable to the intensivist managing early septic shock after the initial emergency department resuscitation and is less applicable to the patient who presents without any prior volume expansion.
The data supporting the validity of CVP or SI as sole predictors of hemodynamic response are conflicting. Some studies demonstrate a difference in CVP between responders and nonresponders [25–27], whereas others indicate no significant difference in CVP between these groups [6,9,11,22,28]. Previous work demonstrated that in a group of 40 septic patients, no patients with an SI less than 1 beat min−1 mm Hg−1 responded to volume expansion [23]. However, our study demonstrated that 12.5% of patients with an SI of 1 beat min−1 mm Hg−1 or less had hemodynamic response to volume expansion. It appears that CVP and SI, by themselves, have significant uncertainty associated with them. We contend that many predictors of hemodynamic response may have a wide range that is clinically indeterminate. Using a composite of 2 (or more) predictors may decrease false positives, false negatives, and indeterminacy. We believe that multimodal assessments are more useful than single-modality assessments.
Strengths of this study include its prospective nature and its patient composition. Some prior studies in this field have limited enrollment to only mechanically ventilated patients, often with chemical paralysis. Before this study, limited information existed about the validity of static indices such as CVP or SI to predict hemodynamic responsive to volume expansion in critically ill, spontaneously breathing patients, or in patients with assisted modes of ventilation. In our study, 23 of 25 patients were spontaneously breathing, a patient composition more representative of current clinical settings.
Limitations of this study include its size. Some may consider the measurement of CI by echocardiography a limitation because the standard method of assessment uses an indwelling pulmonary artery catheter. Routine use of the pulmonary artery catheter has declined because of perceptions of increased risk and lack of therapeutic benefit. Transthoracic echocardiography can accurately assess CI and may be more accurate than the pulmonary artery catheter in some clinical scenarios such as tricuspid regurgitation [19]. Another potential limitation of this study is the use of CVP. In this study, care was taken to ensure the measurement of CVP at end-expiration with appropriate calibration of the transducer height. However, assessment of CVP in clinical practice is often incorrectly done. A parameter that cannot be reproducibly measured in the clinical setting may not be terribly reliable or useful.
We applied a composite measurement of easily obtained parameters (CVP, heart rate, systolic blood pressure) to improve assessment of hemodynamic response. Although neither parameter was useful alone, the combination of CVP and SI appears to be useful in identifying patients who are unlikely to respond to volume expansion. Further studies exploring other methods of assessing response to volume expansion in septic shock may benefit by incorporating CVP and SI into the study protocol before testing the method of interest.
5. Conclusion
In patients with early septic shock and a CVP greater than 8 mm Hg, an SI of 1 beat min−1 mm Hg−1 or less is unlikely to demonstrate an increase in CI from volume expansion. Although a low CVP or a high SI does not necessarily predict a positive hemodynamic response to volume expansion in septic shock, it appears that thresholds exist for these values, beyond which further volume expansion is unlikely to produce improved CI. Given the increasing amount of literature describing increased mortality and morbidity from volume expansion [1–3], we believe that these commonly obtained measurements can be used to help guide volume expansion in early sepsis and may also be of use when designing future study protocols pertaining to volume expansion in septic shock.
Acknowledgment
This study was supported by grants from the Easton Family Fund and the Intermountain Research and Medical Foundation. Dr Brown is supported by a career development award from the National Institute of General Medical Sciences (K23GM094465). We acknowledge Ben Briggs for screening patients, Naresh Kumar for consenting patients, Yao Li for statistical support, and Amanda Borba for administrative support.
Abbreviations
- CVP
central venous pressure
- SI
shock index
- EGDT
early goal–directed therapy
- SIRS
systemic inflammatory response syndrome
- ICU
intensive care unit
- TTE
transthoracic echocardiogram
- VTI
velocity-time integration
- ROC
receiver operating characteristic
- AUC
area under curve
- NPV
negative predictive value
- PPV
positive predictive value
Footnotes
Competing interests: No author has a competing interest in the topic of this study.
Authors’ contributions: M.L. contributed to the study design, enrollment of patients, data acquisition, data analysis, and writing of the manuscript. S.B. contributed to the study design, data analysis, and revision of the manuscript for important intellectual content. J.J. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. E.H. contributed to the study design, and revision of the manuscript for important intellectual content. C.G. contributed to the study design, data analysis, and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jcrc.2012.07.021.
Contributor Information
Michael J. Lanspa, Email: michael.lanspa@imail.org.
Samuel M. Brown, Email: samuel.brown@imail.org.
Eliotte L. Hirshberg, Email: ellie.hirshberg@imail.org.
Jason P. Jones, Email: jason.p.jones@kp.org.
Colin K. Grissom, Email: colin.grissom@imail.org.
References
- 1.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey H, Hall J, Sznajder I, Silverstein M, Wood L. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97(5):1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 3.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal–directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 7.Shippy CR, Appel PL, Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med. 1984;12(2):107–112. doi: 10.1097/00003246-198402000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29(3):352–360. doi: 10.1007/s00134-002-1615-9. [DOI] [PubMed] [Google Scholar]
- 9.Barbier C, Loubieres Y, Schmit C, Hayon J, Ricome JL, Jardin F, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 10.Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867–873. doi: 10.1378/chest.119.3.867. [DOI] [PubMed] [Google Scholar]
- 11.Marx G, Cope T, McCrossan L, Swaraj S, Cowan C, Mostafa SM, et al. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol. 2004;21(2):132–138. doi: 10.1017/s0265021504002091. [DOI] [PubMed] [Google Scholar]
- 12.Pinsky MR. Hemodynamic evaluation and monitoring in the ICU. Chest. 2007;132(6):2020–2029. doi: 10.1378/chest.07-0073. [DOI] [PubMed] [Google Scholar]
- 13.Marque S, Cariou A, Chiche JD, Squara P. Comparison between Flotrac-Vigileo and Bioreactance, a totally noninvasive method for cardiac output monitoring. Crit Care. 2009;13(3):R73. doi: 10.1186/cc7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiel SW, Kollef MH, Isakow W. Non-invasive stroke volume measurement and passive leg raising predict volume responsiveness in medical ICU patients: an observational cohort study. Crit Care. 2009;13(4):R111. doi: 10.1186/cc7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996;14(2):218–225. doi: 10.1016/s0735-6757(96)90136-9. [DOI] [PubMed] [Google Scholar]
- 17.Rady MY, Nightingale P, Little RA, Edwards JD. Shock index: a reevaluation in acute circulatory failure. Resuscitation. 1992;23(3):227–234. doi: 10.1016/0300-9572(92)90006-x. [DOI] [PubMed] [Google Scholar]
- 18.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 19.Colebourn CL, Barber V, Salmon JB, Young JD. The accuracy of diagnostic and haemodynamic data obtained by transthoracic echocardiography in critically ill adults: a systematic review. Journal of the Intensive Care Society. 2008;9(2):7. [Google Scholar]
- 20.McLean AS, Needham A, Stewart D, Parkin R. Estimation of cardiac output by noninvasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care. 1997;25(3):250–254. doi: 10.1177/0310057X9702500307. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 22.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35(1):64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 23.Michard F, Ruscio L, Teboul JL. Clinical prediction of fluid responsiveness in acute circulatory failure related to sepsis. Intensive Care Med. 2001;27(7):1238. doi: 10.1007/s001340100974. [DOI] [PubMed] [Google Scholar]
- 24.McIntyre LA, Hebert PC, Fergusson D, Cook DJ, Aziz A. A survey of Canadian intensivists’ resuscitation practices in early septic shock. Crit Care. 2007;11(4):R74. doi: 10.1186/cc5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider AJ, Teule GJ, Groeneveld AB, Nauta J, Heidendal GA, Thijs LG. Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: a combined hemodynamic and radionuclide study. Am Heart J. 1988;116(1 Pt 1):103–112. doi: 10.1016/0002-8703(88)90256-6. [DOI] [PubMed] [Google Scholar]
- 26.Wagner JG, Leatherman JW. Right ventricular end-diastolic volume as a predictor of the hemodynamic response to a fluid challenge. Chest. 1998;113(4):1048–1054. doi: 10.1378/chest.113.4.1048. [DOI] [PubMed] [Google Scholar]
- 27.Magder S, Bafaqeeh F. The clinical role of central venous pressure measurements. J Intensive Care Med. 2007;22(1):44–51. doi: 10.1177/0885066606295303. [DOI] [PubMed] [Google Scholar]
- 28.Perel A, Minkovich L, Preisman S, Abiad M, Segal E, Coriat P. Assessing fluid-responsiveness by a standardized ventilatory maneuver: the respiratory systolic variation test. Anesth Analg. 2005;100(4):942–945. doi: 10.1213/01.ANE.0000146939.66172.AE. [DOI] [PubMed] [Google Scholar]