Figure 1.
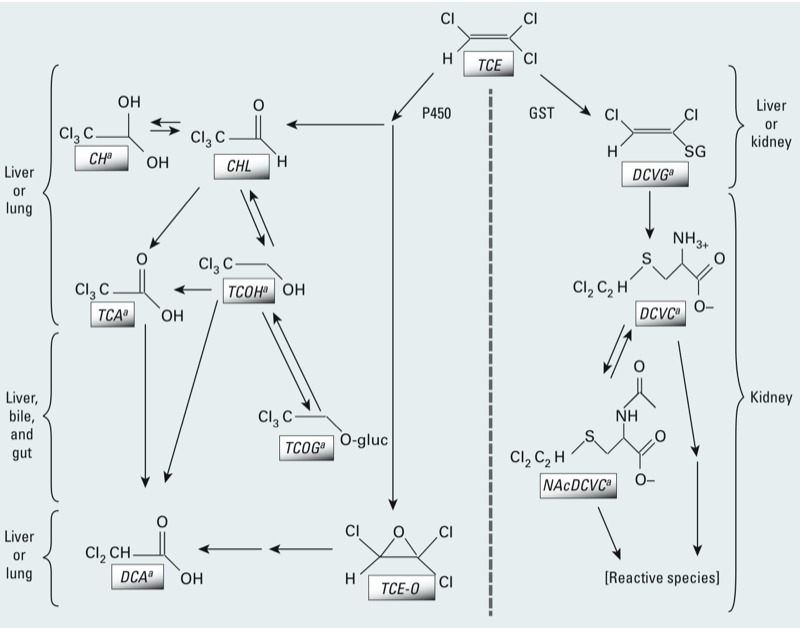
Simplified TCE metabolism scheme. Metabolism of TCE occurs through two main irreversible pathways: oxidation via the microsomal mixed-function oxidase system (i.e., cytochrome P450s; left) and conjugation with GSH by glutathione S-transferases (GSTs; right). Oxidation occurs predominantly in the liver, and to a lesser extent in the lung; the first metabolic products are TCE-oxide (TCE‑O), chloral (CHL), and chloral hydrate (CH), with the latter two quickly transformed to trichloroethanol (TCOH; a reversible reaction) and trichloroacetic acid (TCA). TCOH is glucuronidated to form TCOH-glucuronide (TCOG), which undergoes enterohepatic recirculation (excretion in bile with regeneration and reabsorption of TCOH from the gut). TCA and TCOG are excreted in urine. Further metabolism of TCA and TCOH has not been well characterized but may include dichloroacetic acid (DCA) (Lash et al. 2000a). TCE-O may also form DCA, among other species (Cai and Guengerich 1999). TCE conjugation with GSH in the liver or kidney form dichlorovinyl glutathione (DCVG), which is further processed in the kidney, forming the cysteine conjugate S-dichlorovinyl-L-cysteine (DCVC). DCVC may be bioactivated by beta-lyase or flavin-containing monooxygenases to reactive species (Anders et al. 1988; Krause et al. 2003; Lash et al. 2003), or (reversibly) undergo N-acetylation to the mercapturate N-acetyl dichlorovinyl cysteine (NAcDCVC), which is then excreted in urine or sulfoxidated by CYP3A to reactive species (Bernauer et al. 1996; Birner et al. 1993; Werner et al. 1995a, 1995b).
