Abstract
The prevailing paradigm is that in human rheumatoid arthritis (RA), the accumulation of monocytes and T cells in the joint, mediated in part by such CC chemokine receptors (CCRs) as CCR2 and CCR5, respectively, plays a central role in disease pathogenesis. To further validate this paradigm, we conducted proof-of-principle studies and tested the hypothesis that gene inactivation of Ccr2 or Ccr5 will ameliorate experimental RA. Contrary to our expectations, we found that in two well-established murine models of experimental RA, CCR2 expression in the hematopoietic cell compartment served as a negative regulator of autoantibody production as well as arthritic disease onset, severity, and resolution. In contrast, the RA phenotype in Ccr5-null mice was similar to that of WT mice. Remarkably, the collagen-induced arthritis phenotype of Ccr2–/– mice mimicked closely that of severe human RA, including production of rheumatoid factor, enhanced T cell production, and monocyte/macrophage accumulation in the joints. Our findings demonstrate an essential protective role of CCR2 expression in RA, indicate the existence of alternative receptors responsible for monocyte/macrophage accumulation to inflamed joints, and emphasize the need to clarify carefully the complex effects of the chemokine system in RA before they can be considered as therapeutic targets.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the infiltration of T and B cells, macrophages, and neutrophils into the synovial lining and fluid of the periarticular spaces. Chemokines act as ligands for highly related chemokine receptors that are expressed on distinct leukocyte cell populations, including the cell types recruited to the joint in RA (1). For example, the CC chemokine receptors (CCR) CCR5 (receptor for CCL5, CCL3, and CCL7) and CCR2 (receptor for CCL2, CCL12) are expressed on T cells and monocytes infiltrating the synovium of RA patients, respectively (2, 3). Supporting the role of chemokine receptors in RA pathogenesis, murine experimental arthritis can be partially ameliorated by blocking CCR5 and CCR2 using selective receptor antagonists (4–7). Thus, the prevailing paradigm is that the cellular influx to the inflamed joint in RA is choreographed through the temporal and spatially regulated expression of chemokines and their cognate receptors, prompting the hope of targeting specific members of the chemokine system for therapeutic purposes (1, 8). However, given the plethora of candidate chemokine–chemokine receptors mediating cell recruitment pathways that can potentially contribute to the initiation and resolution of joint inflammation, it has been challenging to determine which receptors, if any, play unique, nonredundant roles in RA pathogenesis.
An especially informative means for evaluating the role of chemokine receptors in RA pathogenesis, including the initiation and resolution of joint inflammation, is the use of mice genetically inactivated for chemokine receptors. Collagen-induced arthritis (CIA) and collagen Ab–induced arthritis (CAIA) are two well-established murine models of human RA that have some distinct features (9, 10). In contrast to CIA, CAIA permits the analysis of both initiation and resolution of joint inflammation (11–15). In CIA, once Ab to type II collagen (CII) is formed, acute arthritis is triggered by Ab binding to collagen in the joints as well as neutrophil activation via Fc receptors, resulting in a disease process in which joint inflammation does not completely subside (9, 10, 14, 16, 17). In contrast, in CAIA the typical disease course involves a short period of acute disease followed by gradual resolution in joint inflammation (13). The CAIA model also permits an analysis of the contribution of the non-T and B cell compartment in the pathogenesis of experimental RA (12, 14, 15, 18–20).
To conduct proof-of-principle studies to elucidate the relative contribution of CCR5 and CCR2 in CIA and CAIA, we genetically inactivated these two receptors in an arthritis-prone murine strain, namely DBA/1J mice. Contrary to our expectations, genetic deletion of Ccr2 was associated with markedly enhanced susceptibility to CIA and CAIA, whereas inactivation of Ccr5 resulted in a CIA phenotype that was indistinguishable from that of WT mice. Our findings provide novel and unpredicted insights into the molecular determinants of RA, and suggest that distinct sets of chemokine receptors are likely to influence the induction and resolution phases of arthritis.
Methods
Mice.
C57BL/6 and DBA/1J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). Ccr2- or Ccr5-KO mice were generated as described previously (21, 22). The KO strains were backcrossed to the inbred DBA/1J mice for either six (Ccr5–/– and Ccr2–/–) or eight (Ccr2–/–) generations. The KO and WT (+/+) mice resulting from mating of the final backcrossed heterozygotes (+/–) were used as breeders to generate the KO and control animals used in this study. Mice were bred and maintained under specific pathogen-free conditions. Some of the phenotypes observed in the Ccr2–/– mice — for example, increased Ab production following antigen (Ag) challenge (refs. 23–25 and this study), enhanced neutrophilic infiltration during inflammation (ref. 25 and this study), defective T cell apoptosis (ref. 26 and this study) — were observed in several disease models and/or diverse Ag challenges as well as in different mice strains (Supplemental Table 1; supplemental material available at http://www.jci.org/cgi/content/full/113/6/856/DC1). For this reason, to elucidate some of the potential mechanisms underlying the CIA phenotype observed in this study, for some selected experiments we used Ccr2–/– mice on a BALB/c or C57BL/6 background (> F6 generation) with appropriate controls from Charles River Laboratories, Inc. (BALB/c; Wilmington, Massachusetts, USA) and The Jackson Laboratory (C57BL/6). The findings of these latter studies are described in Supplemental Tables 3–6 and 8–9. All experimental protocols adhered to IACUC guidelines and were approved by the institutional review boards at the University of Texas at Austin and the University of Texas Health Science Center at San Antonio, Texas, USA.
Induction and assessment of CIA.
Arthritis induction and clinical follow-up for the experiments presented here were performed at the University of Texas at Austin, Texas, USA. Native bovine CII (Elastin Products Company, Inc., Owensville, Missouri, USA) was dissolved in 5 mM acetic acid with gentle stirring at 4°C for 4–8 hours. This solution was then emulsified with an equal volume of complete Freund’s adjuvant containing 5 mg/ml heat-killed Mycobacterium tuberculosis (H37RA; Difco Laboratories, Detroit, Michigan, USA). Groups of 8- to 12-week-old WT, Ccr2–/–, and Ccr5–/– male mice were immunized by intradermal injection at the base of the tail with 150 μg of CII. The mice were boosted 21 days later with an intradermal injection of 150 μg of CII in incomplete Freund’s adjuvant. After the booster injection, mice were monitored two or three times per week for up to 130 days, and the onset and severity of disease were recorded. A clinical severity score assigned for each hindpaw and forepaw was based on the degree of swelling and redness as follows: grade 0, no swelling or erythema; grade 1, obvious swelling and redness in a single digit; grade 2, swelling and erythema in several digits; grade 3, severe swelling in a paw involving all the digits; grade 4, severe swelling with joint rigidity or deformity. The maximum possible clinical score per mouse was 16 (4 paws × 4 grades). To obtain the mean severity score (± SEM), this clinical score for each group of animals at the time point of evaluation was divided by the total number of arthritic mice.
Thioglycollate challenge.
Intraperitoneal injection of thioglycollate, a well-established mouse model of acute peritonitis, was done as described (21). Peritoneal lavage was performed 72 hours after the intraperitoneal injection.
Harvesting of joint and lymph node leukocytes and FACS analysis.
DMEM, RPMI 1640, medium 199, antibiotics, and FCS were purchased from Invitrogen Corp. (Carlsbad, California, USA). All chemicals were from Sigma-Aldrich (St. Louis, Missouri, USA), and Ab’s were from Pharmingen (San Diego, California, USA), with the exception of the biotinylated Ab specific to receptor activator NF-κB ligand (RANKL), which was purchased from R&D Systems Inc. (Minneapolis, Minnesota, USA). Cells infiltrating the joints were harvested by digesting the paws with collagenase for 2 hours at 37°C, and then rigorously homogenizing the tissue using glass slides. The debris was removed by using a 33% Percoll gradient, and the cells were counted and stained with fluorescent-labeled Ab’s according to the manufacturer’s instructions. Leukocytes were also prepared from the lymph nodes draining the inflamed joint. Draining lymph nodes (DLN) were homogenized by gentle crushing between the frosted ends of microscope slides. Erythrocytes were removed from spleen cell suspensions using a red-cell lysis buffer. The leukocytes were incubated with the indicated Ab’s for 15 minutes at 4°C. The level of nonspecific staining was ascertained by using isotype-matched control Ab’s. Cells were analyzed by flow cytometry on a FACScan using Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, California, USA).
Histochemical staining.
The entire joint, together with the overlying skin tissue, was removed and fixed in paraformaldehyde (4% in PBS, pH 7.2), decalcified in 10% EDTA, and embedded in paraffin. Serial sections (5 μm) were made by cryostat sectioning and were stained. For tartrate-resistant acid phosphatase (TRAP) staining, the TRAP solution was prepared as follows: 9.6 mg of naphthol AS-BI phosphate substrate (Sigma-Aldrich) was dissolved in 0.6 ml of N,N-dimethylformamide (Sigma-Aldrich) with 60 ml of 0.2 M sodium acetate buffer (pH 5.0; Sigma-Aldrich) containing 84 mg of fast red-violet LB diazonium salt (Sigma-Aldrich), 58.2 mg of tartaric acid (Sigma-Aldrich), and 240 μl of 10% MgCl2. The mixture was filtered through a 0.22-μm pore-size filter. Slides were incubated for 8 minutes in the staining solution at 37°C in the dark. The slides were then washed with water for 30 minutes and then subjected to counterstaining with methyl green for 5 minutes. Histological analyses was conducted by a pathologist who was blinded to the genetic differences in the experimental groups. Subsequently, a second investigator, also blinded to the study groups, reanalyzed the histopathological sections and took representative photomicrographs.
Radiological assessment of arthritis.
Anteroposterior radiographs of the four limbs were obtained using a cabinet soft X-ray apparatus. An investigator blinded to the experimental groups examined the X-ray films.
Collagen-specific Ab’s.
Anti-CII Ab’s were measured by ELISA as described previously (27). Briefly, the wells of 96-well round-bottomed plates were coated with CII (1–10 μg/ml). After blocking with 1% BSA, the wells were washed with PBS, and serially diluted sera from the mice immunized with CII was added and incubated overnight at 4°C. After washing, wells were incubated for 1 hour at room temperature with alkaline phosphatase–labeled anti-mouse IgG1 mAb’s, anti-mouse IgG2a mAb’s, or anti-mouse IgE mAb’s. Wells were washed and were developed using p-nitrophenyl phosphate (pNPP), and ELISA readings were obtained as described previously (25, 27). Collagen-specific IgE mAb’s were undetectable in these mice.
CAIA.
Ccr2–/– mice and WT littermates (five mice per group) were injected intravenously with 4 mg of CII-specific mAb's, a mixture of four mAb's that recognize individual epitopes within the CB11 fragment of CII (Chemicon International, Temecula, California, USA). After 48 hours had passed, mice received 25 μg LPS (Chemicon International) intraperitoneally and monitored for clinical signs of arthritis twice weekly for 3 weeks. The clinical score was similar to that for CIA.
Rheumatoid factor.
For rheumatoid factor (RF) detection, ELISA plates were coated with purified mouse IgGs overnight at 4°C. All the subsequent steps were carried out at room temperature. Plates were washed to remove unbound IgG, blocked with 4% BSA for 1 hour, and washed again. Serum from immunized animals was added and incubated for 2 hours. After the plates were washed, biotin-labeled anti-mouse IgM mAb’s were added and the plates incubated for 1 hour. After the plates were washed again, streptavidin–alkaline phosphatase (streptavidin-AKP) was added and the plates incubated for 1 hour. After a final wash, the plates were developed using pNPP (substrate for AKP) for 30 minutes and read in an ELISA plate reader.
Determination of Ab’s to single-stranded DNA.
The methods of Warren et al. were adopted (28). Briefly, methylated albumin–coated wells of a 96-well round-bottomed plate were coated with 10 μg/ml of denatured calf thymus DNA and incubated for 90–120 minutes (unless stated otherwise, all incubations were done at room temperature). After washing, the wells were blocked with BSA for at least 1 hour, after which the samples were added and incubated for 2–3 hours at room temperature or overnight at 4°C. Following this incubation, the plates were washed and anti-mouse Ig-AKP conjugated mAb’s were added and the plates incubated for 1 hour. Finally, pNPP substrate was added; the plates were developed for 5–30 minutes and then read in an ELISA plate reader.
Bone marrow transfer.
Recipient DBA/1J (WT or Ccr2–/–) mice or C57BL/6 WT mice received 1,000 cGy of irradiation from a cesium-137 source 8 hours prior to adoptive transfer of 5 × 106 whole bone marrow cells derived from the DBA/1J (WT or Ccr2–/–) or GFP+ C57BL/6 WT mice. In the GFP-transgenic strain, GFP is constitutively expressed in most cells including neural and bone marrow progenitor cells, and it was used as a marker for donor-derived cells. In the C57BL/6 recipients of bone marrow cells from GFP+ C57BL/6 mice, mouse cells were analyzed 10–12 weeks after cell transplantation; 90–95% of the recipient blood or spleen cells were GFP+, suggesting a high degree of chimerism in these adoptive transfer experiments (data not shown). Wild-type DBA/1J mice were lethally irradiated and transplanted with bone marrow cells from WT or Ccr2–/– DBA/1J mice. Additional controls included nontransplanted WT or nontransplanted Ccr2–/– mice. Three months after cell transplantation, the mice were immunized with collagen. Serum samples were collected 2 weeks after the second immunization, and anti-CII Ab levels were measured.
Cell labeling, apoptosis, and in vitro stimulation studies.
To quantify and visualize the fractions of dividing T lymphocytes that were undergoing activation-induced cell death (AICD), we used the methods described by us previously (26) and outlined in detail in the Supplemental Notes.
Ribonuclease protection assay.
Total RNA was isolated from the joints as described by Thornton et al. (29). Briefly, paws from WT or Ccr2-null mice were dissected and snap-frozen in liquid nitrogen, total RNA was then extracted. Ribonuclease protection assays (RPAs) were performed on the total RNA using the RiboQuant Multiprobe RNase Protection Assay Kit (Pharmingen). RNA from the inflamed or noninflamed paws from a given mouse was pooled for analysis. The data are presented as the ratio of the densitometric signals of a housekeeping gene (L32) and the mRNA for the gene of interest.
Ab’s and cell culture for analysis of B cell responses.
To study B cell responses in vitro, the methods of Reimold et al. were used (30). Briefly, mouse splenocytes were plated at 106 cells/ml and stimulated for up to 4 days with anti-CD40 (1 μg/ml), anti-CD40 plus IL-4 (10 ng/ml), LPS (20 μg/ml) alone, or LPS (20 μg/ml) plus IL-4 (10 ng/ml). Subsequently, ELISAs for immunoglobulins (IgG1 and IgG2a) in the culture supernatants were performed. In some experiments, cell proliferation was determined by a nonradioactive cell proliferation assay that depends on the reduction by living cells of tetrazolium salt, MTT, to form a blue formazan product (Promega Corp., Madison, Wisconsin, USA).
Data collection and statistical analyses.
The radiological, histopathological, and some immunological analyses described in this paper were performed 15–30 days after the second immunization with CII, a time point at which the arthritic disease peaked in Ccr2–/– mice, but was not clinically apparent in the WT mice. Data are expressed as the mean ± SEM. The two-tailed Student’s t test for the difference between two means was used for comparing the maximal mean arthritic scores and for comparing anti-CII Ab levels in mouse sera. The Mann-Whitney test was used in instances when the data was not normally distributed.
Results
Increased incidence and accelerated joint destruction in Ccr2-deficient mice during CIA.
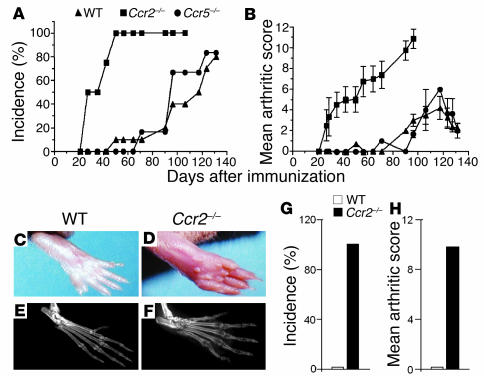
In each of the five separate experiments performed, compared with WT or Ccr5–/– mice, Ccr2–/– mice consistently exhibited a significantly greater incidence and severity of CIA, whereas the CIA phenotypes of Ccr5–/– and WT mice were nearly indistinguishable (Figure 1, A and B). Ccr2–/– mice developed extensive swelling and severe ankylosis in multiple joints, whereas the disease in WT and Ccr5–/– mice was usually restricted to single digits and rarely involved an entire paw (Figure 1, C and D). These findings were corroborated by radiological examination, which revealed involvement of multiple joints, substantial bone destruction, and decrease in bone density, all features characteristic of severe human RA (Figure 1, E and F). Since the lack of CCR5 expression did not modify significantly the course of CIA in DBA/1J mice, no further analyses were performed on the Ccr5–/– animals.
Figure 1.
CIA incidence and severity is increased in Ccr2–/– mice. The data shown in this figure are representative of one of a minimum of three experiments. (A–F) In the experiment shown, Ccr2–/– mice (n = 8), Ccr5–/– mice (n = 6), and WT mice (n = 10) were given primary and booster injections of bovine CII and monitored two to three times per week for incidence and severity of arthritis. All mice were backcrossed six generations into the DBA/1J background. Days after immunization in A and B represent days after the second immunization with CII. (A) The cumulative number of arthritic animals in each group is shown as a percentage of the total number immunized with CII. (B) Arthritic score for each group at each time point was divided by the number of arthritic mice to calculate a mean severity score (± SEM). The maximal arthritic score for Ccr2–/– mice (11.0 ± 0.8) was significantly higher than the scores for WT (3.9 ± 0.7) and Ccr5–/– mice (3.2 ± 1.2) (P < 0.0001). The difference between WT and Ccr5–/– mice was not significant (P = 0.5). Photomicrographs (C and D) and radiographs (E and F) from WT and Ccr2–/– mice depicting severe arthritis and bone destruction and erosion in Ccr2–/– mice. (G) Incidence and (H) severity of arthritis determined 2 weeks after immunization with CII in F8 backcrossed Ccr2–/– (n = 3–4 mice per experiment) and WT mice (n = 3–4 mice per experiment).
To exclude any confounding that might occur as a result of the minimal genetic admixture in the F6 Ccr2–/– DBA/1J mice, we backcrossed these mice an additional two generations. Also, because in these experiments the F6 WT littermates exhibited a benign course compared with Ccr2–/– mice, we sought to determine whether further backcrossing of these littermates would alter their susceptibility to CIA relative to commercially available DBA/1J mice. Remarkably, compared with the F6 backcrossed Ccr2–/– mice, F8 Ccr2–/– mice were even more sensitive to the induction of CIA, and all the F8 Ccr2–/– mice developed arthritis within 14 days of the primary immunization (Figure 1G), whereas the F6 Ccr2–/– mice developed disease only after the booster injection with CII. Notably, at this early time point the disease severity in the F8 Ccr2–/– mice was comparable to that observed 50 days after the booster immunization in the F6 Ccr2–/– mice (Figure 1H). Commercially available DBA/1J mice were only marginally more susceptible than the F8 WT littermates, but the incidence and severity in these commercially obtained control animals remained significantly lower than that observed in the F6 or F8 Ccr2–/– mice (data not shown).
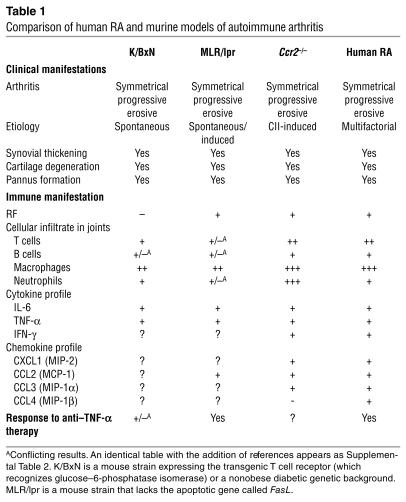
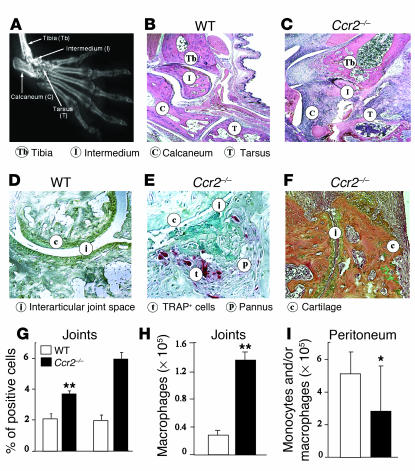
In addition to the clinical and radiographic changes, by day 30 after the second immunization with CII, the Ccr2–/– mice, but not the WT mice, had histopathological features that were highly reminiscent of severe human RA (Table 1 and Supplemental Table 2). The pathology in the joints of these KO mice was characterized by invasion of the pannus tissue into the bone space, destruction of the joint architecture, and severe erosion of cartilage surfaces accompanied by marked infiltration of inflammatory cells, primarily neutrophils and macrophages (Figure 2, A–F). In support of the notion that osteoclasts play an important part in the pathogenesis of focal bone erosion in arthritis (31–34), we observed enhanced osteoclast activity in the joints of Ccr2–/– mice (Figure 2, C and D).
Table 1.
Comparison of human RA and murine models of autoimmune arthritis
Figure 2.
Histopathological and cellular responses in Ccr2–/– mice after induction of CIA or peritonitis. (A) X-ray of the paw of a Ccr2–/– mouse, shown to provide anatomical orientation. The abbreviated names of the bones highlighted in B and C are given in parentheses. Histopathological evaluation of the joints were conducted after (B and C) H&E, (D and E) TRAP (for osteoclasts), and (F) orange G (for enhanced bone visualization) staining. Affected joints derived from WT mice (B and D) show relatively normal articular surfaces, intact joint spaces, and absence of significant periarticular inflammation. Ccr2–/– mice (C, E, and F) showed irregular articular surfaces, collapsed joint space, loss of cartilage, and an increase in connective tissue adjacent to the joint space with abundant osteoclasts stained red by TRAP stain. Representative joints collected 30 days after the second immunization are shown. Note that, at this time point, there was little visual evidence of arthritis in the WT mice and hence the relatively normal histopathology in these mice (see Figure 1B). (F and G) To determine the nature of the cell types recruited, FACS analysis was performed on cells derived from the inflamed joints of four mice from each group. (H) Number of macrophages in inflamed joints. (I) Number of peritoneal monocytes/macrophages 72 hours after thioglycollate injection. *P < 0.05; **P < 0.01. Data shown are representative of one of three experiments. In G–I, the white and black histograms refer to WT and Ccr2-null mice, respectively.
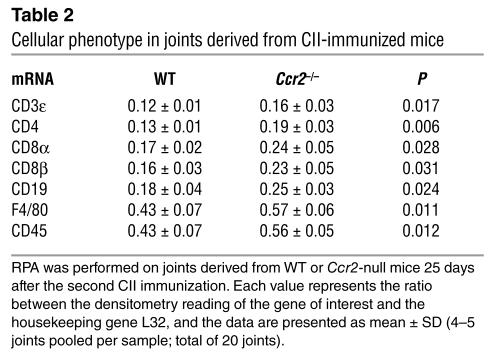
Several leukocyte cell subtypes contribute to human RA pathogenesis (Table 1 and Supplemental Table 2), including neutrophils. Although naive (unmanipulated) WT and Ccr2-null mice had similar numbers of circulating neutrophils, monocytes, and lymphocytes (Supplemental Table 3), after induction of CIA the joints of Ccr2–/– mice had higher cell numbers than WT mice (5.4 ± 1.2 × 106 versus 2.9 ± 1.0 × 106 cells; mean ± SD). FACS analyses revealed an increase in the percentage and total numbers of neutrophils and monocytes and/or macrophages recruited to the Ccr2-null mice (Figure 2, G and H, and data not shown). Mirroring this increased recruitment of monocytes, F4/80 transcripts, a marker characteristic for macrophages, were more abundant in the joints of Ccr2-null mice (Table 2). In addition, the abundance of transcripts characteristic for cell types such as B cells (CD19) and T cells (CD3, CD4, and CD8) were also higher in Ccr2-null mice than in WT mice (Table 2).
Table 2.
Cellular phenotype in joints derived from CII-immunized mice
The enhanced recruitment of monocyte/macrophages to the joints of Ccr2–/– mice was surprising in light of the extensive literature documenting a reduced recruitment of these cell types to inflammatory sites in different disease models tested in these KO mice (Supplemental Table 1). However, concordant with the findings in the literature, after intraperitoneal injection of thioglycollate in the naive DBA/1J mice, lower numbers of monocyte/macrophages were recruited to the peritoneum (Figure 2I). Thus, enhanced or reduced monocyte/macrophage recruitment following genetic inactivation of Ccr2 in DBA/1J mice was clearly highly dependent on the nature of the inflammatory insult. Notably, analogous to our observations here, CCR2-independent recruitment of monocytes and macrophages has also been illustrated in a murine model of idiopathic pulmonary fibrosis (35).
Increased anti-CII Ab, RF, and anti–single-stranded DNA Ab production in Ccr2–/– mice.
CII-specific Ab’s are necessary and sufficient to induce CIA (10, 16, 17), and thus enhanced anti-CII-specific Ab production may serve as a major pathogenic mechanisms underlying the severe CIA phenotype of Ccr2-null mice. Previous studies supported the notion that inactivation of Ccr2 may be associated with increased Ag-specific Ab production. For example, we have previously shown that after Leishmania major infection, Ccr2-null mice in the C57BL/6 genetic background produce higher amounts of L. major–specific Ab’s (25). Additionally, enhanced Ab production was also observed in a murine model of allergen hypersensitivity (refs. 23, 24 and Supplemental Table 1). Here, we extended these findings by showing that in the BALB/c background, inactivation of Ccr2 was also associated with increased L. major–specific Ab’s after intradermal infection (Supplemental Table 4).
We further extend the notion that inactivation of Ccr2 is associated with aberrant Ab responses to different immunogenic challenges and across different genetic backgrounds, since Ccr2–/– DBA/1J mice produced significantly higher levels of anti-CII IgG2a and anti-CII IgG1 compared with WT mice 2 weeks after the primary immunization (Figure 3, A and B). We selected this early time point for analyses of Ab production for two reasons. First, since there is a strong correlation between anti-CII Ab titers before the onset of arthritis and subsequent arthritic severity (36), we surmised that elevated levels of anti-CII Ab at this early time point would support a possible link between aberrant Ab production and the increased disease severity observed in Ccr2-null mice. Second, this early time point we posited would potentially limit the confounding that might occur once clinical arthritis is evident, since the disease per se may act as a feedback mechanism to increase autoantibody production, and therefore the finding of abnormal Ab production during the later stages of disease may not necessarily represent a primary immune abnormality. In this light, our findings suggest that CCR2-dependent processes are associated with the magnitude of anti-CII Ab production, and may be associated with the more severe CIA phenotype observed in Ccr2-null mice. However, we cannot exclude the possibility that clinically undetectable joint disease during the very early phases of CIA also contributed to the enhanced anti-CII Ab production in Ccr2–/– null mice (37).
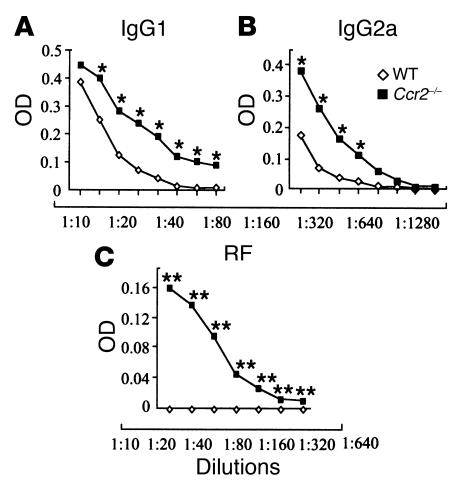
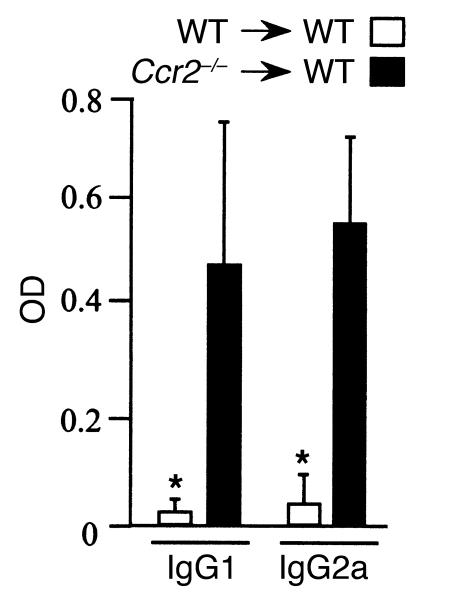
Figure 3.
Elevated levels of autoantibodies 2 weeks after CII immunization in Ccr2–/– mice, as shown by OD measurements in serially diluted serum samples. (A) IgG1 and (B) IgG2a against collagen (mean of three WT and four Ccr2-deficient mice); *P < 0.05. (C) RF was measured in the sera of three WT and four Ccr2–/– mice; **P < 0.0001. Collagen-specific IgG quantification was performed in several experiments, and Ccr2–/– mice consistently had higher IgG levels than the WT littermates before arthritic disease was clinically evident, and those levels remained elevated after the arthritic disease started (data not shown).
We next determined whether the production of other autoantibodies was also increased after CII immunization in Ccr2–/– mice. Since among all biomarkers currently known for human RA, autoantibodies against IgG (e.g., RF) have consistently been shown to be the best predictor of disease severity (38–40), we measured RF 2 weeks after primary immunization in WT and Ccr2–/– mice. After induction of CIA, RF was undetectable in serum from WT mice, a finding concordant with previous studies (10, 17, 39, 41, 42). However, RF was detected in the sera of Ccr2–/– mice (Figure 3C), thus reproducing one of the characteristic features of human RA, and notably, a feature of only a handful of other rodent models of RA (Table 1, and refs. 38–40, 42–46). Furthermore, compared with WT mice, higher levels of circulating anti–single-stranded DNA (anti-ssDNA) Ab’s were detected in the Ccr2–/– mice (in WT mice, OD = 0.049 versus Ccr2-null mice, OD = 0.165; P = 0.001).
Given that an aberrant production of autoantibodies, especially anti-CII Ab’s, appeared to be a major feature of the severe CIA phenotype in Ccr2–/– mice, we next tested the hypothesis that this was secondary to an intrinsic defect(s) at the level of Ccr2-null B cells that was present in these mice before CII immunization (e.g., enhanced proliferation, increased Ab production, accelerated maturation). Several lines of evidence suggested that Ccr2-null B cells before induction of CIA were normal. First, in vitro experiments showed that after stimulation of splenocytes with anti-CD40 (1 μg/ml), anti-CD40 plus IL-4 (10 ng/ml), LPS (20 μg/ml), or LPS (20 μg/ml) plus IL-4 (10 ng/ml), DBA/1J WT and Ccr2–/– mice splenocytes produced similar amounts of Ab (data not shown) and had similar proliferation rates (Supplemental Table 5). Second, following B cell receptor stimulation using IgM F(ab′)2 fragments, mature B cells derived from the spleens of WT and Ccr2–/– mice appeared to have comparable phosphorylation levels of tyrosine kinases, including that of Syk, a critical proximal kinase that initiates all B cell receptor–dependent signaling pathways (47) (Supplemental Notes and data not shown). Third, the percentage of pro- (B220+CD43+IgM–), pre- (B220+CD43–IgM–), immature (B220+IgM+IgD–), and mature (IgM+IgD+B220hi) B cell populations in the spleens, bone marrow, and peritoneal cavities of naive (nonimmunized) WT and Ccr2–/– mice was similar, as were B-1 cells (B220+IgM+CD5+) (Supplemental Table 6). Taken together, these findings suggest that B cell responses in vitro as well as the B cell development in Ccr2-null mice do not differ significantly from WT mice.
Since we found no gross defects at the level of Ccr2–/– B cells prior to CII immunization to account for the severe CIA phenotype, we next determined the contribution of T cell–mediated immune responses to enhanced Ab production and joint inflammation: cytokine production at the local (joint) and the secondary lymphoid organ level and intrinsic abnormalities in the Ccr2-null T cells (i.e., Th cell polarization and AICD).
Cytokine responses in Ccr2–/– mice following CII immunization.
There is considerable evidence to suggest that in DBA/1J mice, CIA is a Th1-mediated inflammatory disease (5, 10, 48, 49). Typically, Th1 responses are evaluated by determining cytokine levels from the spleen or DLN of mice with CIA. However, recent studies indicate that the cytokine responses in the peripheral lymphoid organs reflect the immune reactivity against the mycobacterial component of the adjuvant, whereas the cytokine responses in the joints may mirror more accurately the immune response against collagen and not the adjuvant (9, 50, 51). Additionally, we reasoned that although several cytokines could be upregulated systemically, elucidation of those cytokines that drive the inflammatory process at the level of the joints (i.e., local microenvironment) may be especially informative regarding the Th mechanisms underlying the severe joint inflammation that we observed in Ccr2-null mice. For these reasons, we first determined the cytokine levels by RPA in the inflamed and noninflamed joints of Ccr2–/– mice.
Consistent with the notion that high levels of IFN-γ (9) may be a pathogenic feature of severe joint destruction in CIA, there was abundant expression of this cytokine in the inflamed, but not the noninflamed joints of Ccr2–/– mice derived from the same animal, whereas in contrast, Th2 cytokines (i.e., IL-4, IL-5, and IL-13) were undetectable (Table 3). Furthermore, the expression of CCL3, a chemokine associated with Th1 responses (52), as well as other proinflammatory chemokines, were also upregulated in the inflamed joints of Ccr2-null mice (Table 3). Notably, in light of the increased recruitment of neutrophils to the Ccr2-null joints, the expression of macrophage inflammatory protein 2 (MIP-2), a chemokine that is fairly specific for recruitment of neutrophils, was also elevated (53–55).
Table 3.
Inflammatory mediators in joints derived from CII-immunized Ccr2–/– mice
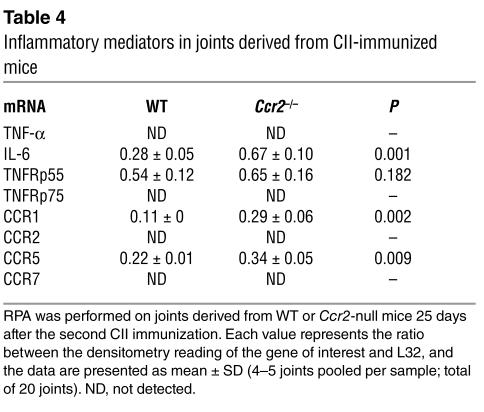
In addition to these Th1/Th2 cytokines, we determined the expression profile of additional inflammatory mediators such as IL-6 and TNF-α and chemokine receptors in the joints of WT and Ccr2-null mice (Table 4). Notably, an enhanced expression of IL-6, CCR1, and CCR5 was detected in Ccr2-null mice, but there were no differences in the expression levels of TNF-α or its receptors p55 and p75 between KO and WT mice (Table 4). These findings, taken together with the data shown in Table 3, suggest that a biased localized Th1 response and increased IL-6 production in Ccr2–/– mice may play a significant role in driving the inflammatory processes in the joint, and that the enhanced expression of several chemokines and their cognate receptors may account for the nature of the cellular infiltrate in the inflamed joints. At least at this early time point, the TNF system did not appear to contribute significantly to joint pathology; however, it is conceivable that at other time points during CIA this gene system may also play a key role.
Table 4.
Inflammatory mediators in joints derived from CII-immunized mice
To extend the aforementioned studies, we also determined the spontaneous as well as Ag (CII)-specific IFN-γ production from the spleens and DLN of WT and Ccr2-null mice 25 days after the second immunization, a time point at which joint inflammation was evident in the KO mice. Spontaneous IFN-γ (mean ± SD) production in the DLN (498 ± 284 pg/ml) and spleens (25 ± 33 pg/ml) was evident only in the Ccr2-null mice. Additionally, the Ag-induced IFN-γ levels in the DLN were also higher in Ccr2–/– (3,500 ± 147 pg/ml) compared with WT (1.7 ± 3 pg/ml; P < 0.001) mice. Ag-induced IFN-γ production in the spleens of Ccr2–/– (1,830 ± 803 pg/ml) and WT (1,174 ± 1,890 pg/ml) mice was similar. Interestingly, although IL-4 expression was not detected in the inflamed joints of Ccr2-null mice, the Ag-specific IL-4 production from the spleens (WT: 12 ± 12 pg/ml; Ccr2–/–: 182 ± 81 pg/ml; P < 0.05) and the DLN (WT: 2.3 ± 1.3 pg/ml; Ccr2–/–: 66 ± 64 pg/ml; P < 0.08) was also increased in Ccr2-null mice compared with WT mice. Thus, in the inflamed joints a Th1 response may be a pathogenic variable leading to joint destruction, whereas the systemic Th0 responses may reflect the responses to collagen as well as Mycobacterium Ag’s (9).
One possibility to account for this Th1-bias in the relevant local microenvironment was that this Th1 bias was secondary to the genetic background — that is, it was specific to the DBA/1J background in which CIA was elaborated. An alternative possibility is that this Th1 bias is due to the specific nature of the immunogen. To differentiate between these two possibilities, we resorted to the well-characterized model system used to characterize Th1/Th2 polarization in vivo, that is, the infection model with L. major. DBA/1J mice are normally resistant to L. major infection (56); however, we found that after intradermal infection of the ears of WT and Ccr2-null mice with L. major, these Ccr2-null mice in a DBA/1J background were highly susceptible to infection (increased ear swelling, higher parasite burdens) and increased Th2 (IL-4) levels in the DLN (Supplemental Table 7, and data not shown). Notably, susceptibility to L. major is characteristically associated with an increase in Th2 cytokines. Thus, it would appear that the Th response in DBA/1J mice is secondary to the nature of the immunological challenge: in CIA it is associated with a Th1 response, whereas in L. major infection it is associated with a Th2 response. Further supporting the notion that Ccr2-null mice can develop a Th1-biased response, we found that in a graft-versus-host-disease (GVHD) model, Ccr2-null mice in a C57BL/6 background develop an enhanced Th1 response associated with a poor GVHD outcome (26).
Normal Th polarization and proliferation but decreased AICD in Ccr2–/– T cells.
We next determined whether abnormalities intrinsic to Ccr2-null T cells that are present before CII immunization could provide insights into the mechanisms leading to a severe CIA phenotype. In light of the observation that there was increased IFN-γ cytokine production in the inflamed joints as well as the DLN of Ccr2-null mice, we determined whether Ccr2-null Th cells had a higher intrinsic ability to produce Th1 cytokines after T cell receptor stimulation and under Th1-polarizing conditions. We found that highly purified naive T cells derived from WT and Ccr2–/– mice were comparable in their ability to differentiate and produce canonical Th1 or Th2 cytokines under appropriate polarizing conditions (Supplemental Table 8).
In light of the findings of normal Th1 and Th2 skewing in vitro, we hypothesized that either abnormal proliferation or T cell AICD rates in naive (unchallenged) Ccr2–/– mice may contribute to both increased cytokine and T cell–dependent Ab production. Although WT and Ccr2–/– DBA/1J mice had similar rates of T cell divisions, Ccr2-null splenocytes had a significant reduction in the percentage of apoptotic T cells after stimulation with anti-CD3 (Figure 4A). A similar phenotype was recapitulated when Ccr2–/– or WT T cells were stimulated with anti-CD3 plus anti-CD28. After 72 hours of stimulation with anti-CD3/CD28, the percentage of CD4+ Ccr2-null apoptotic cells was significantly lower than in WT T cells (data not shown). These findings are concordant with our recent observation that Ccr2-null T cells derived from KO mice on a C57BL/6 background also had impaired AICD (26). This defect in AICD did not appear to be secondary to a gross defect in T cell receptor–dependent signaling, since the tyrosine phosphorylation patterns of critical kinases such as PLC-γ–1, ZAP-70, and MAPK1/2 induced by T cell receptor cross-linking using anti-CD3 Ab in highly purified T cells derived from WT and Ccr2–/– mice was similar (Supplemental Notes and data not shown).
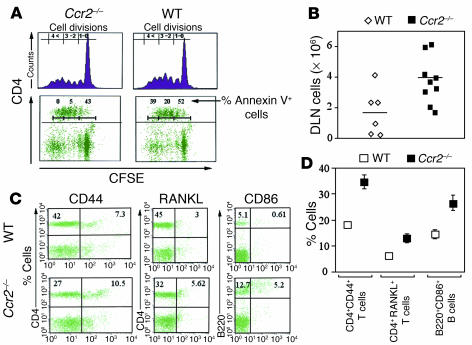
Figure 4.
Higher accumulation of activated T and B cells in vivo, and decreased AICD in vitro in Ccr2–/– mice. (A) Quantitative analysis of proliferation and apoptosis of WT or Ccr2-null splenocytes derived from naive mice after stimulation with anti-CD3. carboxy-fluorescein succinimidyl ester–labeled (CFSE-labeled) splenocytes from WT and Ccr2–/– mice were stimulated with soluble anti-CD3 antibody (2 μg/ml), and cellular proliferation was assessed by FACS after 72 hours. CFSE-labeled cells were also stained for CD4 and Annexin V, and the percentage of CD4+AnnexinV+ cells in each cell division was determined. The number of generations is noted in the upper left of each panel. Data shown in this panel are representative of at least three separate experiments derived from two animals per group. Single-cell suspensions were prepared from arthritic joints DLN derived from WT or Ccr2–/– mice 30 days after the second immunization with CII. Cell numbers (B) and activation markers on T and B cells (C and D) in the DLN of WT and Ccr2–/– mice are depicted. The cells were counted and stained with the fluorescent-labeled Ab’s shown in B and C. In D, the percentage of activated T cells (CD4+/CD44+) or (CD4+/RANKL+) or B cells (B220+/CD86+) within the total T or B cell population is shown. The findings shown in A–C are representative of three separate experiments, and the data depicted are from three WT and five Ccr2–/– mice.
If the aforementioned in vitro findings of reduced AICD in Ccr2-null T cells were potentially contributing to the CIA phenotype in Ccr2-null mice, we surmised that we should observe an increase in the accumulation of activated T and B cells after induction of CIA in Ccr2–/– mice and be able to transfer the disease phenotype to WT animals by adoptive transfer of Ccr2-null whole bone marrow cells and/or purified T cells.
Potential contributions of reduced AICD in Ccr2–/– mice to the CIA phenotype: accumulation of activated T and B cells.
CII-immunized Ccr2–/– mice exhibited prominent lymphadenopathy. Consistent with this observation, the DLN of Ccr2-null mice had a greater cellular content (Figure 4B) that was associated with increased total numbers and percentages of activated T cells (CD4+CD44+ cells; Figure 4, C and D). Notably, we also found that the lymph nodes of Ccr2–/– mice had a higher accumulation of T cells that expressed the osteoclastogenic factor RANKL (CD4+RANKL+) that might potentially account for the severe bone loss observed in these mice (Figures 1F and 4, C and D) (32, 57). In addition, the DLN of Ccr2–/– mice had nearly three times as many B cells and, more importantly, the number of activated B cells was four times higher in these mice compared with WT mice (Figure 4, C and D, and data not shown). Thus, the increased cellularity in the DLN of Ccr2–/– mice was accompanied by an enhanced activation state of the T and B cells. This accumulation in the number of activated T cells was observed only after CII immunization, since prior to CIA induction the percentage of naive as well as activated and memory T cells in Ccr2–/– and WT mice was comparable (Supplemental Table 9).
Potential contributions of reduced AICD in Ccr2–/– mice to the CIA phenotype: adoptive-transfer studies.
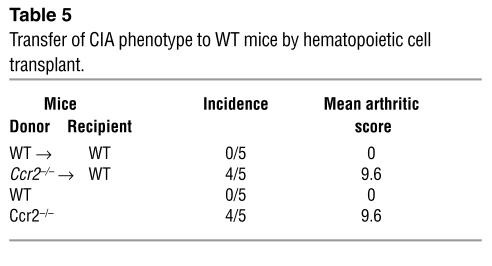
Bone marrow cells from naive WT or Ccr2–/– mice were adoptively transferred into lethally irradiated WT mice. At 3 months after transplant, a time point at which the hematopoietic system of the recipients was reconstituted (95% chimerism as described in Methods), we immunized these mice with CII. Wild-type mice reconstituted with WT bone marrow cells did not develop arthritis 3 months after the second immunization with CII (Table 5). In contrast, WT mice transplanted with Ccr2–/– bone marrow cells developed frank arthritis within 30 days after the second immunization with clinical arthritic scores similar to those observed in untransplanted Ccr2–/– mice immunized with CII (Table 5). In addition, compared with WT mice that were recipients of WT bone marrow cells, higher levels of anti-collagen IgGs were detected in WT recipients of Ccr2–/– bone marrow cells (Figure 5). Taken together, these findings indicated that WT DBA/1J mice adoptively transferred with Ccr2–/– cells had a CIA phenotype that mimics closely that observed in untransplanted Ccr2–/– DBA/1J mice, demonstrating that the expression of CCR2 in the hematopoietic compartment is a critical determinant of CIA.
Table 5.
Transfer of CIA phenotype to WT mice by hematopoietic cell transplant.
Figure 5.
Transplantation of CCR2-null hematopoietic cells is sufficient to transfer the CIA phenotype to WT animals. Bone marrow cells derived from WT and Ccr2–/– mice were transplanted into WT recipients that had been lethally irradiated (1,000 cGy). Three months after transplantation, the protocol to induce CIA was initiated as described in the Methods section. Collagen-specific Ab’s determined in the serum of transplanted animals two weeks after the second immunization.
Whether the CIA phenotype can be adoptively transferred readily from CII-immunized donor mice into a syngeneic host (not allogeneic SCID) by transplantation of only purified T cells is not clear, since only very few studies have demonstrated that this is possible (58). Most adoptive-transfer studies have used CII-specific T cell clones or whole splenocytes followed by reimmunization of the mice with CII to induce disease (59–63). Nevertheless, we attempted to transfer the CIA phenotype by adoptive transfer of purified T cells using the protocols of Delgado et al. (58). WT or Ccr2-null DBA/1J mice were immunized with CII, and 14 days after immunization, T cells purified from their spleens were transferred intravenously into naive DBA/1J mice; the recipient mice were not subsequently challenged with CII. Up to 100 days after transplant we observed no phenotypic changes in the recipient mice.
Ccr2-null mice are highly susceptible to CAIA.
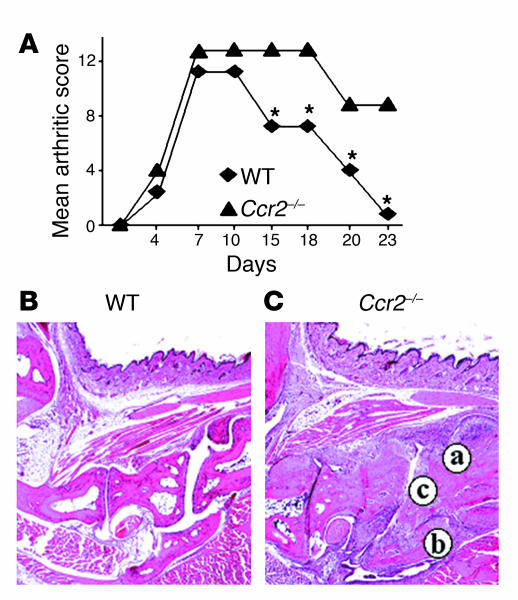
The combined injection of anti-CII Ab’s on day 0 and LPS on day 2 induced arthritis in both WT and Ccr2–/– mice (Figure 6A). The first sign of slight swelling was detected around day 4, and paw swelling with redness reached a maximum on days 6–7. As demonstrated in previously published reports, we found that the CAIA-affected animals developed swollen, red, and ankylosed joints (12, 20). The swelling and associated other arthritic changes continued until day 10–12 in WT and Ccr2–/– mice. However, after this time point we observed a differential response in the resolution phase of the disease: signs of arthritis gradually decreased after day 12 and were minimal by day 23 in WT mice, whereas in Ccr2–/– mice signs of severe arthritis persisted, suggesting a delay in the processes that lead to a resolution in inflammation. Histological analysis confirmed these findings, since 23 days after injection of the arthritogenic Ab’s the joints of the WT mice had minimal signs of disease, whereas the joints of Ccr2–/– mice had prominent signs of chronic arthritis with pannus formation, and destructive bone and cartilage erosion, predominantly of the distal joints (Figure 6, B and C). Collectively, these findings indicated a possible requirement of CCR2 expression for the resolution of the ongoing inflammatory processes during CAIA.
Figure 6.
Enhanced CAIA phenotype in Ccr2–/– mice. (A) Mean arthritic score after induction of CAIA was determined in WT and Ccr2–/– mice. Histopathological analyses (H&E staining) of joints from (B) WT and (C) Ccr2–/– mice. Significant joint destruction was evident in Ccr2–/– mice compared with WT mice. Letters indicate (a) chronic inflammatory infiltrate and extensive synoviocyte proliferation; (b) loss of interarticular space; (c) cyst formation. *P < 0.001.
Discussion
Although animal models may not reproduce all the features of human RA, they can help understand normal inflammatory and immune responses during RA pathogenesis or serve as vehicles to test novel therapeutic agents. The information obtained from these models and human studies informs the current view of RA pathogenesis in which an initial T cell–dependent phase, characterized by the development of T cell Ag-specific autoreactivity leading to the generation of autoantibodies, is followed by a T cell–independent phase characterized by Ab deposition, complement activation, leukocyte infiltration into the synovium, and tissue destruction (9, 10, 13, 16). Within this conceptual framework, the findings obtained in the Ccr2-null mice after induction of CIA suggest that, contrary to our expectations, chemokine-dependent processes may play important roles during this T cell–dependent (e.g., AICD and production of autoantibodies) and T cell–independent phases of RA pathogenesis (e.g., leukocyte infiltration).
Furthermore, our results also suggest the intriguing possibility that in CIA either CCR2 is dispensable for the migration of monocyte/macrophages into inflamed joints, or CCR2– subpopulations of monocyte/macrophages accumulate in inflamed joints and contribute to disease pathogenesis. The existence of monocyte populations that can be subdivided based on their CCR2 expression (positive and negative) levels has recently been demonstrated by elegant studies from the Littman lab (64). Although these authors suggest that the CCR2– monocyte cell populations may migrate only to the noninflamed sites to become resident macrophages, it is conceivable that in the context of specific disease processes and cellular compartments such as CIA, or even human RA, this CCR2– leukocyte population may infiltrate into the inflamed joints. For example, although 84% of circulating monocytes in normal subjects expressed CCR2, only 76% of monocytes expressed CCR2 in patients with RA, but more importantly, even fewer monocytes in the synovial fluid from RA patients expressed CCR2 (24%), suggesting that the majority of the monocytes recruited to RA joints may be CCR2– (65). This reduced detection of CCR2– monocytes was thought not to be secondary to ligand-mediated downregulation of the receptor, since the expression of other chemokine receptors such as CCR5 on this cell population in the synovial fluid (47%) was higher than in the peripheral circulation (17%) (65).
Another important feature of the CIA phenotype observed in Ccr2-null mice was the presence of RF. Even though the sensitivity and specificity of RF for identification of patients with RA is relatively low compared with the more recently discovered autoantibodies, several studies show that RF predisposes to the development of RA (38, 43, 44). Increased levels of RF in symptom-free individuals greatly increase the risk of acquiring RA, and high titers predict persistent radiographic damage in inflammatory polyarthritis (38, 43, 44). Moreover, RF levels are also elevated in relatives of RA patients and RA-prone rats, providing evidence of an independent genetic control of RF production (38, 43, 44). Thus, identification of the mechanisms that promote RF production specifically, and enhanced Ab production in general, in Ccr2-null mice could be helpful in understanding the pathogenesis of RA.
Ccr2
-null mice are one of the most intensely scrutinized chemokine receptor KOs studied to date (Supplemental Table 1). Diverse phenotypes in these mice have been described after infectious, inflammatory, autoimmune, transplant, and other challenges. Ascribing a precise mechanism that is applicable to each of these phenotypes or common mechanistic features has been a daunting task. Initial studies suggested that a prominent Th2 skewing and/or impaired monocyte/macrophage migration was a common denominator for several of the phenotypes observed in mice genetically inactivated for Ccr2. However, these two characteristics are not universal features of the phenotype of Ccr2-null mice in the diverse disease models examined (Supplemental Table 1). Thus, the role of CCR2 in the immune response appears to be more variable than initially anticipated, and as illustrated by the studies here, the immunological outcome following its inactivation appears to be dependent on the nature of the antigenic challenge.
A provocative question that comes to mind is whether abnormal T cell AICD in Ccr2-null mice constitutes an important pathogenic feature of the CIA phenotype in these mice. We have insufficient evidence to conclusively link decreased AICD and enhanced susceptibility to CIA, including T cell and B cell activation and increased anti-CII Ab production in this model. Nevertheless, it is notable that inhibition of T cell apoptosis has been associated previously with both RA and CIA (66, 67). In addition, RA patients have a subset of CD4+CD28– T cells in the peripheral blood that frequently undergoes clonal expansion and is resistant to apoptosis, and additionally, Bcl-2 and related antiapoptotic proteins are upregulated in the T cells of RA patients (66). In this light, we speculate that impaired T cell AICD observed in Ccr2–/– mice may contribute to an increased autoreactivity by enhancing T cell help to B cells for Ab production in CIA.
It is noteworthy that our in vivo proof-of-principle studies in Ccr5-null mice do not support the prevailing notion that CCR5 is a central player in RA pathogenesis (3, 68–71). This finding should be interpreted in light of the observation that CCR5 is not the only chemokine receptor whose expression is elevated in RA. For example, invariably both CCR5 and CXCR3 are elevated in RA (68, 69, 72). Furthermore, although in the CIA model, chemical antagonism of CCR5 leads to partial protection against the disease, it is important to note that the antagonist used in these studies blocks not only CCR5 but also CXCR3 (7), making it difficult to ascribe the protective effects solely to the antagonism of CCR5. Supporting our findings that CCR5 may play a redundant role in RA pathogenesis are the observations of Santiago et al., who showed that the leukocyte chemoattractant activity in RA synovial fluid was not dependent on the expression of CCR5 (73).
In summary, our findings emphasize the importance of conducting proof-of-concept studies using chemokine receptor knockouts to delineate the full repertoire of their redundant and nonredundant functions in diverse disease states, and emphasizes that the nature of the Th phenotype, at least in Ccr2-null mice, may be highly dependent on the nature of the antigenic challenge. Our findings of increased monocyte recruitment, enhanced susceptibility to arthritis, and autoantibody production following genetic inactivation of Ccr2 suggest that additional research is required before therapeutic manipulation of distinct members of the chemokine system can be safely accomplished.
Supplementary Material
Acknowledgments
We thank H. Kulkarni for statistical analyses, K. Czarra, N. Sato, and G. Gomez for skillful technical assistance, O. Willmon, M. Dudley, and B.S Castillo for their help in the preparation of this manuscript, and A.S. Ahuja for continuing support. M.P. Quinones thanks A.W. McCurdy for enduring support. This work was supported by an NIH grant (AI-48644) to S.S. Ahuja and an American Heart Association beginning grant-in-aid to W.A. Kuziel. S.K. Ahuja is a recipient of the Elizabeth Glaser Scientist Award (Pediatric AIDS Foundation) and a Burroughs Wellcome Clinical Scientist Award in Translational Research.
Footnotes
William A. Kuziel’s present address is: Autoimmune and Inflammatory Diseases, Protein Design Labs Inc., Fremont, California, USA.
William A. Kuziel and Seema S. Ahuja contributed equally to this work.
Nonstandard abbreviations used: activation-induced cell death (AICD); alkaline phosphatase (AKP); antigen (Ag); chemokine receptor (CCR); collagen Ab–induced arthritis (CAIA); collagen-induced arthritis (CIA); draining lymph nodes (DLN); graft-versus-host-disease (GVHD); macrophage inflammatory protein (MIP); monocyte chemoattractant protein (MCP); p-nitrophenyl phosphate (pNPP); receptor activator NF-κB ligand (RANKL); rheumatoid arthritis (RA); rheumatoid factor (RF); ribonuclease protection assay (RPA); single-stranded DNA (ssDNA); tartrate-resistant acid phosphatase (TRAP); type II collagen (CII).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Gerard C, Rollins BJ. Chemokines and disease. Nat. Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 2.Bruhl H, et al. Surface expression of CC- and CXC-chemokine receptors on leucocyte subsets in inflammatory joint diseases. Clin. Exp. Immunol. 2001;126:551–559. doi: 10.1046/j.1365-2249.2001.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mack M, et al. Predominance of mononuclear cells expressing the chemokine receptor CCR5 in synovial effusions of patients with different forms of arthritis. Arthritis Rheum. 1999;42:981–988. doi: 10.1002/1529-0131(199905)42:5<981::AID-ANR17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Gong JH, Ratkay LG, Waterfield JD, Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J. Exp. Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arend WP, Dayer JM. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33:305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 6.Carter PH. Chemokine receptor antagonism as an approach to anti-inflammatory therapy: ‘just right’ or plain wrong? Curr. Opin. Chem. Biol. 2002;6:510–525. doi: 10.1016/s1367-5931(02)00351-4. [DOI] [PubMed] [Google Scholar]
- 7.Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol. Lett. 1997;57:117–120. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 8.Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin. Immunol. 2003;15:15–21. doi: 10.1016/s1044-5323(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 9.Luross JA, Williams NA. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103:407–416. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony DD, Haqqi TM. Collagen-induced arthritis in mice: an animal model to study the pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 1999;17:240–244. [PubMed] [Google Scholar]
- 11.Loutis N, Bruckner P, Pataki A. Induction of erosive arthritis in mice after passive transfer of anti-type II collagen antibodies. Agents Actions. 1988;25:352–359. doi: 10.1007/BF01965042. [DOI] [PubMed] [Google Scholar]
- 12.Terato K, et al. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22:137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- 13.Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am. J. Pathol. 2003;163:1827–1837. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki N, et al. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J. Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- 15.Stuart JM, Dixon FJ. Serum transfer of collagen-induced arthritis in mice. J. Exp. Med. 1983;158:378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin. Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- 17.Stuart JM, Watson WC, Kang AH. Collagen autoimmunity and arthritis. FASEB J. 1988;2:2950–2956. doi: 10.1096/fasebj.2.14.3053308. [DOI] [PubMed] [Google Scholar]
- 18.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 19.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 2003;25:79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- 20.Terato K, et al. Induction of arthritis with monoclonal antibodies to collagen. J. Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 21.Kuziel WA, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuziel WA, et al. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis. 2003;167:25–32. doi: 10.1016/s0021-9150(02)00382-9. [DOI] [PubMed] [Google Scholar]
- 23.Blease K, et al. Enhanced pulmonary allergic responses to Aspergillus in CCR2–/– mice. J. Immunol. 2000;165:2603–2611. doi: 10.4049/jimmunol.165.5.2603. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, et al. Enhanced airway Th2 response after allergen challenge in mice deficient in CC chemokine receptor-2 (CCR2) J. Immunol. 2001;166:5183–5192. doi: 10.4049/jimmunol.166.8.5183. [DOI] [PubMed] [Google Scholar]
- 25.Sato N, et al. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao AR, et al. CC chemokine receptor 2 expression in donor cells serves an essential role in graft-versus-host disease. J. Immunol. 2003;171:4875–4885. doi: 10.4049/jimmunol.171.9.4875. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, et al. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J. Immunol. 2000;164:4340–4347. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 28.Warren RW, Caster SA, Roths JB, Murphy ED, Pisetsky DS. The influence of the lpr gene on B cell activation: differential antibody expression in lpr congenic mouse strains. Clin. Immunol. Immunopathol. 1984;31:65–77. doi: 10.1016/0090-1229(84)90190-9. [DOI] [PubMed] [Google Scholar]
- 29.Thornton S, et al. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin. Immunol. 2002;105:155–168. doi: 10.1006/clim.2002.5227. [DOI] [PubMed] [Google Scholar]
- 30.Reimold AM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 31.Goldring SR, Gravallese EM. Pathogenesis of bone lesions in rheumatoid arthritis. Curr. Rheumatol. Rep. 2002;4:226–231. doi: 10.1007/s11926-002-0069-y. [DOI] [PubMed] [Google Scholar]
- 32.Gravallese EM. Bone destruction in arthritis. Ann. Rheum. Dis. 2002;61(Suppl. 2):ii84–ii86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redlich K, et al. Osteoclasts are essential for TNF-α–mediated joint destruction. J. Clin. Invest. 2002;110:1419–1427. doi:10.1172/JCI200215582. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldring SR, Gravallese EM. Mechanisms of bone loss in inflammatory arthritis: diagnosis and therapeutic implications. Arthritis Res. 2000;2:33–37. doi: 10.1186/ar67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore BB, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J. Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 36.Williams PJ, Jones RH, Rademacher TW. Correlation between IgG anti-type II collagen levels and arthritic severity in murine arthritis. Autoimmunity. 1998;27:201–207. doi: 10.3109/08916939808993831. [DOI] [PubMed] [Google Scholar]
- 37.Holmdahl R, Jonsson R, Larsson P, Klareskog L. Early appearance of activated CD4+ T lymphocytes and class II antigen-expressing cells in joints of DBA/1 mice immunized with type II collagen. Lab. Invest. 1988;58:53–60. [PubMed] [Google Scholar]
- 38.Scott DL. Prognostic factors in early rheumatoid arthritis. Rheumatology (Oxford). 2000;39(Suppl. 1):24–29. doi: 10.1093/oxfordjournals.rheumatology.a031490. [DOI] [PubMed] [Google Scholar]
- 39.Chen PP, Fong S, Carson DA. Rheumatoid factor. Rheum. Dis. Clin. North Am. 1987;13:545–568. [PubMed] [Google Scholar]
- 40.Newkirk MM. Rheumatoid factors: host resistance or autoimmunity? Clin. Immunol. 2002;104:1–13. doi: 10.1006/clim.2002.5210. [DOI] [PubMed] [Google Scholar]
- 41.Klareskog L. What can we learn about rheumatoid arthritis from animal models? Springer Semin. Immunopathol. 1989;11:315–333. doi: 10.1007/BF00197310. [DOI] [PubMed] [Google Scholar]
- 42.Izui S. Pathogenic role of rheumatoid factors. Ryumachi. 1994;34:171–172. [PubMed] [Google Scholar]
- 43.Wollheim FA. Predictors of joint damage in rheumatoid arthritis. APMIS. 1996;104:81–93. doi: 10.1111/j.1699-0463.1996.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 44.Wernhoff P, Olofsson P, Holmdahl R. The genetic control of rheumatoid factor production in a rat model of rheumatoid arthritis. Arthritis Rheum. 2003;48:3584–3596. doi: 10.1002/art.11342. [DOI] [PubMed] [Google Scholar]
- 45.Richardson C, Emery P. Laboratory markers of disease activity. J. Rheumatol. Suppl. 1996;44:23–30. [PubMed] [Google Scholar]
- 46.Jefferis R, Mageed RA. The specificity and reactivity of rheumatoid factors with human IgG. Monogr. Allergy. 1989;26:45–60. [PubMed] [Google Scholar]
- 47.Clark MR, Massenburg D, Zhang M, Siemasko K. Molecular mechanisms of B cell antigen receptor trafficking. Ann. N. Y. Acad. Sci. 2003;987:26–37. doi: 10.1111/j.1749-6632.2003.tb06030.x. [DOI] [PubMed] [Google Scholar]
- 48.Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J. Immunol. 2000;164:6495–6502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- 49.Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur. J. Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 50.Mauri C, Williams RO, Feldman M. Down regulation of Th1 mediated pathology in experimental arthritis by stimulation of the Th2 arm of the immune response. Arthritis Rheum. 2003;48:839–845. doi: 10.1002/art.10832. [DOI] [PubMed] [Google Scholar]
- 51.Stasiuk LM, Abehsira-Amar O, Fournier C. Collagen-induced arthritis in DBA/1 mice: cytokine gene activation following immunization with type II collagen. Cell. Immunol. 1996;173:269–275. doi: 10.1006/cimm.1996.0277. [DOI] [PubMed] [Google Scholar]
- 52.Chiu BC, et al. Cytokine-chemokine networks in experimental mycobacterial and schistosomal pulmonary granuloma formation. Am. J. Respir. Cell Mol. Biol. 2003;29:106–116. doi: 10.1165/rcmb.2002-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Call DR, et al. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock. 2001;15:278–284. doi: 10.1097/00024382-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 54.Lomas JL, et al. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock. 2003;19:358–365. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Bozinovski, S., et al. 2003. Innate immune responses to lipopolysaccharide in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am. J. Physiol. Lung Cell. Mol. Physiol. doi:10.1152/ajplung.00275.2003. [DOI] [PubMed]
- 56.Shaw BA, Grayson JB, Petersen EA. Experimental murine infection with the diffuse cutaneous leishmaniasis strain, Isabel. Ann. Trop. Med. Parasitol. 1987;81:9–14. doi: 10.1080/00034983.1987.11812083. [DOI] [PubMed] [Google Scholar]
- 57.Gravallese EM, Galson DL, Goldring SR, Auron PE. The role of TNF-receptor family members and other TRAF-dependent receptors in bone resorption. Arthritis Res. 2001;3:6–12. doi: 10.1186/ar134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 59.Holmdahl R, Klareskog L, Rubin K, Larsson E, Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand. J. Immunol. 1985;22:295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 60.Kadowaki KM, Matsuno H, Tsuji H, Tunru I. CD4+ T cells from collagen-induced arthritic mice are essential to transfer arthritis into severe combined immunodeficient mice. Clin. Exp. Immunol. 1994;97:212–218. doi: 10.1111/j.1365-2249.1994.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor PC, Plater-Zyberk C, Maini RN. The role of the B cells in the adoptive transfer of collagen-induced arthritis from DBA/1 (H-2q) to SCID (H-2d) mice. Eur. J. Immunol. 1995;25:763–769. doi: 10.1002/eji.1830250321. [DOI] [PubMed] [Google Scholar]
- 62.Taylor PC, Chu CQ, Plater-Zyberk C, Maini RN. Transfer of type II collagen-induced arthritis from DBA/1 to severe combined immunodeficiency mice can be prevented by blockade of Mac-1. Immunology. 1996;88:315–321. doi: 10.1111/j.1365-2567.1996.tb00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrow PK, Thoss K, Katenkamp D, Brauer R. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: role of CD4+ and CD8+ T cells. Immunol. Invest. 1996;25:341–353. doi: 10.3109/08820139609059316. [DOI] [PubMed] [Google Scholar]
- 64.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 65.Katschke KJ, Jr, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 66.Salmon M, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J. Clin. Invest. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taneja V, et al. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J. Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- 68.Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin. Immunol. 2001;98:39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- 69.Qin S, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki N, et al. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int. Immunol. 1999;11:553–559. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 71.Zapico I, et al. CCR5 (chemokine receptor-5) DNA-polymorphism influences the severity of rheumatoid arthritis. Genes Immun. 2000;1:288–289. doi: 10.1038/sj.gene.6363673. [DOI] [PubMed] [Google Scholar]
- 72.Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2000;43:765–774. doi: 10.1002/1529-0131(200004)43:4<765::AID-ANR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 73.Santiago B, et al. The chemoattraction of lymphocytes by rheumatoid arthritis - synovial fluid is not dependent on the chemokine receptor CCR5. Rheumatol. Int. 2002;22:107–111. doi: 10.1007/s00296-002-0203-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.