Abstract
Background:
Lipoprotein (a) [Lp (a)] is an established risk marker of coronary artery disease which is independent from other risk factors.
Objective:
The aim was to address the association between Lp (a) and CAD risk in North Indians. To evaluate whether high levels of lipoprotein (a) [Lp (a)] is a predictor of risk and is related to the severity of CAD.
Materials and Methods:
This was a cross-sectional study done on 360 patients presenting with chest pain. Coronary angiography revealed CAD in 270 patients and 90 patients without CAD. Lipoprotein (a) level, lipid profile, fasting blood glucose, anthropometric and clinical parameters were analyzed.
Results:
Lipoprotein (a) 21.0 mg/dL is associated with the presence of coronary lesions (P = 0.0001). A highly significant difference in Lp (a) levels was observed between normal coronaries vs. single-vessel disease, double-and triple-vessel disease ( P < 0.0001). Body mass index (BMI) was significantly raised in CAD group compared to normal coronary.
Conclusion:
Multivariate analysis found that Lp (a) was considered an independent predictor for severity of CAD and Lp (a) levels 21.0 mg/dL are associated with severe patterns of coronary atherosclerosis.
Keywords: Angiography, atherosclerosis, lipoprotein (a), triglyceride
INTRODUCTION
Cardiovascular diseases in India cause 3 million deaths/y, accounting for 25% of all mortality.[1] Moreover, research on Indian Asians living abroad indicates a 40% higher risk of ischemic heart disease (IHD) mortality than that for Europeans.[2]
Traditional risk factors like smoking, hypertension, and diabetes are reported to account for only 50% of the prevalence and severity of the disease.[3] The atherogenic dyslipidemic profile, especially mild to marked elevation of apo-B-containing lipoproteins, such as very low-density lipoproteins (VLDL), VLDL-remnants, intermediate-density lipoproteins (IDL), and low-density lipoproteins (LDL), and low levels of high-density lipoproteins (HDL)[4–6] appears to promote enhanced arterial cholesterol deposition and accelerate the progression of atherosclerotic disease.
Despite the use of new and effective pharmacological drugs to lower plasma lipid concentration, cardiovascular diseases continue to be the main cause of death in Western countries.[7,8]
Lipoprotein (a) (Lp (a)) has emerged as a powerful genetic risk factor for coronary artery disease (CAD).[9–11]
Lp (a) presents a lipid composition which is similar to the composition of LDL, but with a different protein content, since it presents the apolipoprotein (a) or apo (a) linked to apolipoprotein B by disulfide bridges.[12]
In advanced atherosclerosis, Lp (a) is an independent risk factor not dependent on LDL. Lp (a) represents a coagulant risk of plaque thrombosis.[13] ApoA contains domain that are very similar to plasminogen. The main function of plasminogen is to dissolve fibrin blood clots. Lp (a) accumulates in the vessel wall and inhibits binding of plasminogen to cell surface. This inhibition of Lp (a) also promotes proliferates of smooth muscle cells. These unique features of Lp (a) suggest that it causes generation of clots and atherosclerosis.
This study aimed at investigating the existence of changes in serum levels of Lp (a) and other risk factors in patients diagnosed with CAD established on angiography, comparing them to individuals with normal angiogram to correlate possible changes with the severity of the lesion.
MATERIALS AND METHODS
Study population
A cross-sectional study was done on 360 consecutive patients (300 male, 60 female) who underwent coronary angiography between June 2008 to October 2010. The study was approved by the Institutional ethics committee. Subjects were informed on the objectives and procedure of the study and informed consent taken. The consent form was both in English and Hindi language. Demographic profile, socioeconomic status, personal habits and disease risk factor history was recorded. Blood pressure (BP) was measured at the ward before the patients were sent to catheterization laboratory. Hypertension was defined as history of hypertension or taking antihypertensive drugs at referral or systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg. Those patients with known history of diabetes mellitus (DM) or with fasting glucose higher than 126 mg/dl were labeled as DM.
Inclusion criteria patients for angiography with history of chest pain, angina, previously diagnosed myocardial infarction were enrolled. Exclusion criteria subject with diseases such as nephrotic syndrome, acute or chronic renal failure, thyroid disorders, acute infections, stroke and diabetic ketoacidosis were excluded.
Angiography evaluation
Coronary angiography was performed using the Judkins technique. Coronary angiography results were evaluated byinterventional cardiologists who were blinded to the serum Lipoprotein (a) analysis.
In coronary angiography, CAD is defined as the presence of at least a > 50% stenosis of major coronary arteries (left anterior descending, left circumflex, or right coronary arteries) or their major branches (diagonal, obtuse marginal, posterior descending, or posterior left ventricular arteries).
Blood collection and analysis
Fasting blood samples were collected after 10 to 12 h before cardiac catheterization. Samples were taken in sterile tubes, centrifuged at 3000 rpm for 10 min at 4°C, and then stored at −80°C until assayed. Fasting blood glucose (FBG) level, serum total cholesterol, HDL-cholesterol (HDL-C), serum triglyceride and LDL-cholesterol (LDL-C) were estimated by standard methods.
Lipoprotein (a) estimation
Lp (a) was measured by agglutination due to an antigen – antibody reaction between Lp (a) in a sample and anti-Lp (a) antibody absorbed to latex particles.The assay range is approximately 3-90 mg/dl (Randox.Lab. Ltd U.K). Higher values of Lp (a) were again rechecked by diluting the sample by normal saline and results were standardized.
RESULTS
The mean age of the patients was 54.31(±8.35) years ranging from 30-65 years. Among 360 patients, 270 patients (75%) had angiographically proven CAD and 90 (25%) had normal coronary arteries (non-CAD group). The systolic and diastolic blood pressure was significantly ( P <0.001) higher in CAD patients as compared to non-CAD patients. The body mass index (BMI), total leukocyte count and fasting glucose were significantly ( P <0.0001) higher in CAD patients as compared to non-CAD patients.
There was a highly significant ( P <0.0001) difference in the level of triglycerides between CAD (182.76 ± 43.86 mg/dl) and non-CAD (124.14 ± 44.01 mg/dl) patients. A highly significant difference was observed in the level of VLDL between CAD (36.03 ± 9.19mg/dl) and non-CAD (24.68 ± 8.89 mg/dl) patients. However, the HDL was significantly ( P < 0.0001) lower in CAD (30.88 ± 7.52 mg/dl) patients as compared to non-CAD (40.04 ± 9.27 mg/dl) patients. The lipoprotein (a) level was significantly higher in CAD (48.73 ± 23.85 mg/dl) patients than in non-CAD patients (18.95 ± 11.15 mg/dl)[Table 1].
Table 1.
Comparison of clinical and biochemical parameters between coronary artery disease and non.CAD patients and as defined by angiography
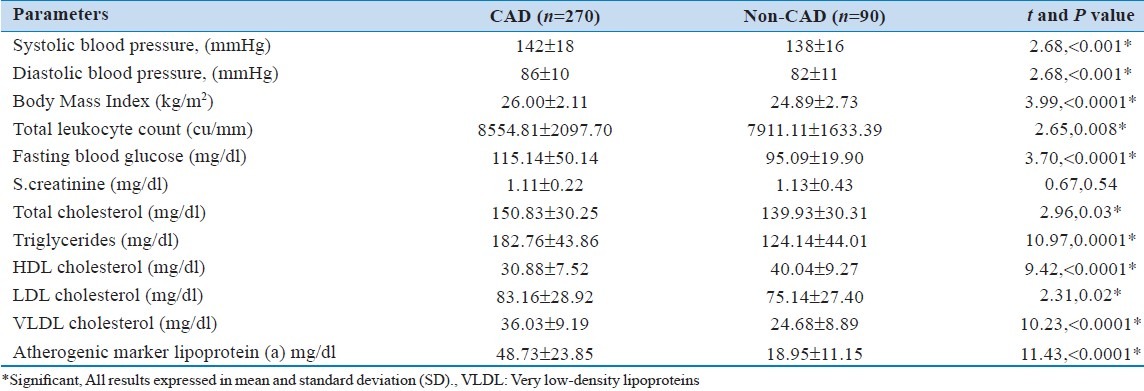
Comparison between diabetics and non-diabetics in diseased vessel patients
The BMI, total leukocyte count, total cholesterol, triglyceride and VLDL levels were almost similar in both diabetic and non-diabetic diseased vessel patients ( P > 0.05). However, fasting glucose was significantly ( P <0.0001) higher in diabetic diseased vessel patients as compared to non-diabetic diseased vessel patients. Similarly, serum creatinine was also significantly (P = 0.02) higher in diabetic than non-diabetic diseased vessel patients. The level of lipoprotein (a) was significantly ( P <0.0001) higher in diabetic (60.20 ± 27.72 mg/dl) than non-diabetic (44.72 ± 20.99 mg/dl) diseased vessel patients [Table 2].
Table 2.
Comparison of body mass index, hematological and biochemical parameters between diabetics and non-diabetics in diseased vessel patients
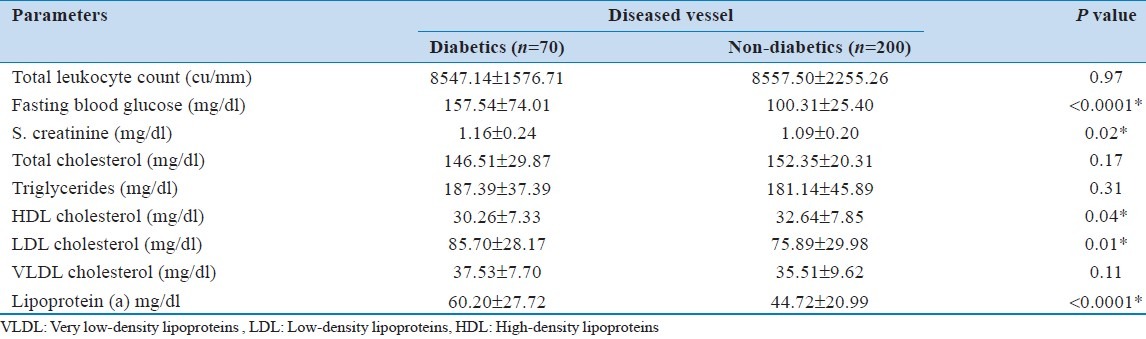
Comparison of parameters by vessel grades
Total leukocyte count was significantly different (P = 0.001) among the vessel grades. The Bonferroni pairwise comparison test showed that total count was significantly (P = 0.001) different between vessel Grade 1 and 3. The total cholesterol, HDL and LDL were insignificantly different among the vessel grades ( P > 0.05). However, triglyceride (P = 0.01) and VLDL (P = 0.006) were significantly different among the vessel grades. The Bonferroni pairwise comparison test showed that both triglyceride and VLDL were significantly (P = 0.01) different between vessel Grade 1 and 3 [Table 3].
Table 3.
Comparison of hematological and biochemical parameters by vessel grades
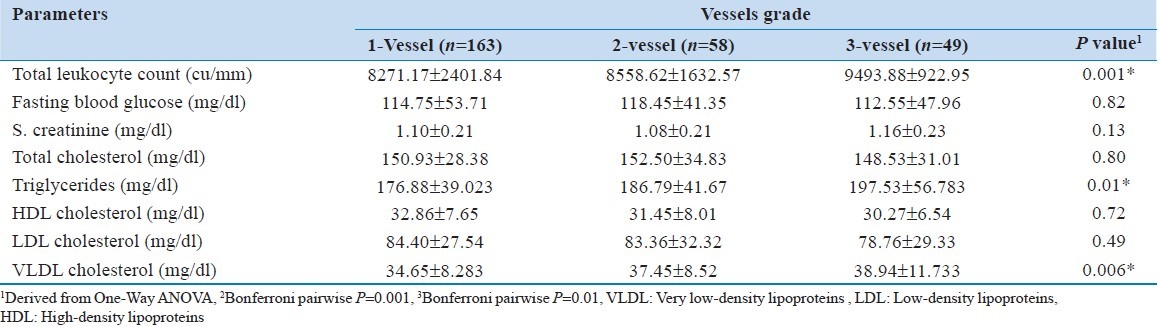
The lipoprotein (a) level was significantly ( P < 0.0001) different among normal and different vessel grades. The level was also significantly ( P < 0.0001) different from Grade 1 to 2 and 3. This was also significantly (P = 0.01) different between vessel Grade 2 and 3 [Table 4].
Table 4.
Lipoprotein (a) level in normal coronary and diseased vessels
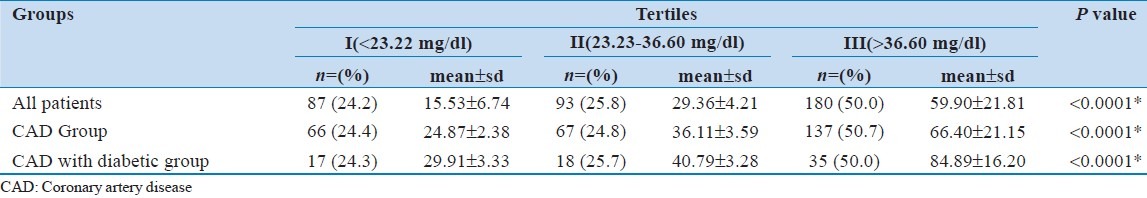
Half (50%) of the patients were in Tertile III followed by Tertile II (25.8%) and Tertile I (24.2%) with mean lipoprotein level of 59.90 ± 21.81 mg/dl, 29.36 ± 4.21 mg/dl and 15.53 ± 6.74 mg/dl respectively. An almost similar pattern was observed for CAD and CAD with diabetic patients [Table 5].
Table 5.
Lipoprotein (a) levels in different tertiles
DISCUSSION
In our study Lp (a) levels correlated positively with severity of atherosclerosis. The most relevant finding was that Lp (a) levels 21.0 mg/dL were not only associated with the presence of coronary disease but also with the severity of the coronary atherosclerosis.
A trend towards an association between higher Lp (a) levels and severe patterns of coronary atherosclerosis has been reported.[14] Our results indicate that Lp (a) levels 21.0 mg/dL are associated with the presence of coronary disease, even when cardiovascular risk factors and specific treatments (statins and/or aspirin) were taken into account.
Results show that a high level of TG (150 mg/dl) is strongly associated with greater extent of CAD. which is in accordance with the documented epidemiologic observations reported by Melissa Austin that hypertriglyceridemia commonly occurred in CAD patients.[15] Triglyceride concentrations (130 mg/dl) had a positive predictive value of 70% to identify individuals at risk for insulin resistance[16] and insulin resistance and hypertriglyceridemia may contribute together to the increased risk of CAD in hypertension.[17] Relation between Lp (a) and coronary disease should be a constant concern and object of investigation, especially in normocholesterolemic individuals.
In conclusion, this study confirms the usefulness of Lp (a) and other risk factors to predict the severity of coronary atherosclerosis, suggesting that Lp (a) levels should be determined in patients with CAD, especially in normolipidemic individuals. Although all patients with hypertension and CAD require aggressive risk modification, the subgroup with high levels of Lp (a) and TG may designate multiple-vessel involvement and probably needs close clinical surveillance.
There are limitations to our study in that enrolled subject might not be representative of the entire population with CAD as subjects recruited were referred for coronary angiography in tertiary care hospitals.
ACKNOWLEDGMENTS
We would like to acknowledge the support of the clinical biochemistry staff SGPGIMS, Lucknow for laboratory work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Mukherjee AK. India's health: Today and tomorrow. J Indian Med Assoc. 1995;93:312–5. [PubMed] [Google Scholar]
- 2.Balarajan R. Ethnicity and variations in mortality from coronary heart disease. Health Trends. 1996;28:45–51. [Google Scholar]
- 3.Gupta S, Johnamm A. Chlamydia pneumoniaeand CHD coincidence, association or causation. BMJ. 1997;314:1779–81. [Google Scholar]
- 4.Taskinen MR. Diabetic dyslipidaemia: From basic researchto clinical practice. Diabetologia. 2003;46:733–49. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 5.Williams KJ, Tabas I. Lipoprotein retention-andclues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–40. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 6.Le NA, Walter MF. The role of hypertriglyceridemia in atherosclerosis. Curr Atheroscler Rep. 2007;9:110–5. doi: 10.1007/s11883-007-0006-7. [DOI] [PubMed] [Google Scholar]
- 7.Breslow JL. Cardiovascular disease burden increases, NIH funding decreases. Nat Med. 1997;3:600–1. doi: 10.1038/nm0697-600. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E. Shattuck lecture cardiovascular medicine at the turn of the millennium: Triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Collins R, Peto R. Lipoprotein (a) and coronary heart disease: A meta-analysis of prospective studies. Circulation. 2000;102:1082–5. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 10.Von Eckardstein A, Schulte H, Cullen P, Assmann G. Lipoprotein (a) further increases the risk of coronary events in men with high global cardiovascular risk. J Am Coll Cardiol. 2001;37:343–9. doi: 10.1016/s0735-1097(00)01126-8. [DOI] [PubMed] [Google Scholar]
- 11.Luc G, Bard JM, Arveiler D, Ferrieres J, Evans A, Amouyel P, et al. Lipoprotein (a) as a predictor of coronary heart disease: The PRIME study. Atherosclerosis. 2002;163:377–84. doi: 10.1016/s0021-9150(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 12.Koschinsky ML. Lipoprotein (a) and the link between atherosclerosis and trombosis. Can J Cardiol. 2004;20:37–43. [PubMed] [Google Scholar]
- 13.Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor; A novel link between lipoproteins and thrombosis. Blood. 2001;98:2980–7. doi: 10.1182/blood.v98.10.2980. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzman RA, Cox ID, Poloniecki J, Crook R, Seymour CA, Kaski JC. Elevated plasma lipoprotein (a) is associated with coronary artery disease inpatients with chronic stable angina pectoris. J Am Coll Cardiol. 1998;31:1260–6. doi: 10.1016/s0735-1097(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 15.Austin MA. Epidemiology of hypertriglyceridemia and cardiovascular disease. Am J Cardiol. 1999;83:13F–6F. doi: 10.1016/s0002-9149(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–9. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 17.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential Hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003;88:2399–403. doi: 10.1210/jc.2003-030087. [DOI] [PubMed] [Google Scholar]


