Abstract
Some local cultivars and wilds of Iwateyamanashi (Pyrus ussuriensis var. aromatica) that grows wild in Northern Tohoku, Japan have good aromatic fruit. Iwateyamanashi may be valuable germplasms as a donor of odor compounds in breeding of Japanese pear (Pyrus pyrifolia), because almost all Japanese pear cultivars have faint odor. Fruits odors from a local cultivar ‘Sanenashi’, a wild accession (i0830) in Iwateyamanashi, cultivars of ‘Kosui’ and ‘La France’ were characterized at first with comparative Aroma Extract Dilution Analysis (AEDA). Application of AEDA, based on Gas chromatography/Olfactometry analysis (GC/O), on the odor concentration prepared from ‘Sanenashi’ indicated the presence of 33 odor-active compounds including methyl and ethyl esters, aldehydes and alcohol. The eleven odor compounds from 16 accessions of Iwateyamanashi showed various combinations and wide range of odor concentrations by Principal Component Analysis (PCA). Especially 2 accessions of local cultivar ‘Natsunashi’ plotted in the highly ethyl ester group might be useful for Japanese pear breeding.
Keywords: pear, Pyrus, Iwateyamanashi, odor-active compound, GC/O, AEDA
Introduction
Fruit odor and texture are important factors for fruit quality and consumer satisfaction in European pear cultivars. Typical European pear has a buttery juicy texture with rich odor (Rapparini and Predieri 2003). However modern Japanese pear cultivars have only faint odor compare to European pear cultivars. Even though the production of European pear (P. communis L.) cultivar ‘La France’ has been increasing in Japan, it is still less than 9% of the total pear production in Japan (Statistical Yearbook of Ministry of Agriculture, Forestry and Fisheries, Japan 2011, http://www.maff.go.jp/e/tokei/kikaku/nenji_e/85nenji/index.html). Rich odor with ‘European pear’ might promote its consumption in Japan. One of Japanese cultivar ‘Kaori’ (Hiratsuka No. 16) derived from the cross between ‘Shinko’ × ‘Kosui’ has exceptionally fruity odor flesh. The production of ‘Kaori’ has been gradually increasing in Japan (Kotobuki 2000). Japanese pear breeding with rich fruit odor might stimulate new demands in both the production and consumption of Japanese pear cultivar.
We reported recently, by means of morphological and molecular analyses, that Iwateyamanashi (P. ussuriensis var. aromatica, syn. of Michinokunashi) is endemic in Northern Tohoku, Japan (Iketani et al. 2010, Iketani and Katayama 2012, Katayama and Uematsu 2006, Katayama et al. 2007). As indicated by its Latin name, ‘aromatica’, good odor is an obvious characteristic of Iwateyamanashi fruit (Nakai 1919). Wide aromatic diversity of some wild accessions and local cultivars of Iwateyamanashi was revealed by sensory evaluation from the Iwateyamanashi germplasm collections at Kobe University (Matsumura et al. 2011). Different apple-like, European pear-like and apricot-like or sweet and fruity odors were recognized in some accessions in Iwateyamanashi. It is considered that Iwateyamanashi is a valuable genetic resource as donor of odors for Japanese pear breeding. Local cultivars, e.g. ‘Sanenashi’, ‘Sotoorihime’, ‘Kinkoji’ and ‘Natsunashi’ are considered to have originated from Iwateyamanashi, but these are not cultivated or utilized at all now (Asami 1921, Kajiura and Sato 1990, Katayama and Uematsu 2006, Kikuchi 1946). ‘Sanenashi’ was favoured by people from the Edo era (1600–1867) because of its good odor, taste and small seedless core, it was widely propagated by grafting (Katayama et al. 2007). ‘Natsunashi,’ called ‘summer pear’, with a strong, sweet odor and high acidity was also characterized as one of the earliest ripening pears in Japan (Matsumura et al. 2011).
The odor compounds of pear have been reported to consist of over 300 components including hydrocarbons, aldehydes, alcohols, esters, ketones and sulphur compounds (Suwanagul and Richardson 1998). However, studies involving the odor compounds of pear are still relatively few compared to those concerned with apple, strawberry, peach and banana (Morton and Macleod 1990). The odor compounds of European pear have been studied extensively (Rapparini and Predieri 2003). In some European pears, butyl acetate and hexyl acetate are the major acetate esters (Rapparini and Predieri 2002, Shiota 1990), while acetate esters are minor components in Asian pear cultivars such as ‘Ya li’, ‘Choujuurou’, ‘Hosui’, ‘Kosui’ and ‘Shinko’ (Horvat et al. 1992). Horvat et al. (1992) described the diversity of the relative amounts of individual compounds from 5 Asian pear cultivars. Imayoshi et al. (1995) reported ethyl acetate, ethyl butanoate and ethyl hexanoate as the major esters emitted by the Chinese white pear ‘Ya li’, which produced an ester profile quite different from that of European pears (Shiota 1990, Suwanagul and Richardson 1998). Acetate esters such as butyl acetate and hexyl acetate were detected as dominant components in Chinese sand and xinjiang pear cultivars ‘Seuri’ and ‘Kuerle fragrant pear’ (Chen et al. 2006, Takeoka et al. 1992). Aldehydes such as hexanal and trans-2-hexanal, α-farnesene, esters such as ethyl butyrate and ethyl hexanoate are important components of characteristic fruit odors in 7 Japanese pear cultivars (Horvat et al. 1992, Yamamoto 1997). Despite the existence of ussrian pear cultivars with good odor fruit, such as ‘Nanguo li’ in China, there has not been an intensive study of odor-active compounds in ussurian pear and even less study on Iwateyamanashi in Japan.
To characterize the odors of fruit from Iwateyamanashi collected from the Northern part of Japan, odor concentrations were extracted and subjected to gas chromatography-mass spectrometry (GC-MS) analysis in this study. Odor-active compounds were identified by Gas chromatography/Olfactometry analysis (GC/O) and evaluated using the Aroma Extract Dilution Analysis (AEDA) and sensory evaluation methods. In AEDA, serial dilutions of a odor extract are evaluated by GC/O to provide a flavor dilution (FD) factor, the highest dilution at which an odor-active compound could be detected. The FD factor reflects odor value (concentration/odor threshold) for an odor-active compound, which may then be applied to compare relative odor intensities with other odor-active compounds. A wide range of odor-active compounds from Iwateyamanashi were defined by PCA.
Materials and Methods
Plant materials
An aromatic local cultivar ‘Sanenashi’, (i0147) a wild accession (i0830) of Iwateyamanashi originating from Nothern Tohoku, Japan, a European pear cultivar ‘La France’ and a Japanese pear cultivar ‘Kosui’ were used to GC/O and AEDA analyses in this study (Fig. 1). For PCA, all Iwateyamanashi trees without ‘Sanenashi’ were maintained by top grafting at genebank of wild pear in Food Resources Research and Education Center, Kobe University (Fig. 1 and Table 1). Fruit of ‘Sanenashi’ (i0147) was sampled from native tree in Iwate in Japan. Fourteen local cultivars and 2 wild accessions of Iwateyamanashi, 5 cultivars of Japanese pear, a Chinese white pear cultivar, an European pear cultivar and an apple were used.
Fig. 1.
Fourteen local cultivars and 2 wild accessions of Iwateyamanashi used in this study. a. ‘Natsunashi’ (i0009), b. ‘Sanenashi’ (i0147), c. ‘Jinashi’ (i0187), d. i0218, e. ‘Hanbeinashi’ (i0471), f. i0605, g. i0830, h. i0868, i. i0959, j. i0981, k. i0193, l. ‘Wayamanashi’ (i1104), m. i1003, n. i1115, o. ‘Natsunashi’ (i1302) and p. ‘Natsunashi’ (i1701).
Table 1.
Plant materials used in this study
| Accession name | Species | Location | Category of Individuals | Harvest date |
|---|---|---|---|---|
| Natsunashi i0009a | Pyrus ussuriensis var. aromatica | Kunohe, Iwate, Japan | Local | 17 July 2007 |
| Natsunashi i1302a | ” | Kuji, Iwate, Japan | Local | 30 July 2007 |
| Natsunashi i1701a | ” | Ninohe, Iwate, Japan | Local | 24 July 2007 |
| Sanenashi i0147b | ” | Iwaizumi, Iwate, Japan | Local | 30 Oct. 2007 |
| Jinashi i0187a | ” | Hanamaki, Iwate, Japan | Local | 27 Aug. 2007 |
| i0193a | ” | Shizukuishi, Iwate, Japan | Local | 27 Aug. 2007 |
| i0218a | ” | Ichinoseki, Iwate, Japan | Local | 26 Oct. 2007 |
| Hanbeinashi i0471a | ” | Yuda, Iwate, Japan | Local | 8 Sept. 2007 |
| i0605a | ” | Kawai, Iwate, Japan | Wildc | 16 Sept. 2007 |
| i0830a | ” | Morioka, Iwate, Japan | Wildc | 16 Sept. 2007 |
| i0868a | ” | Kuji, Iwate, Japan | Local | 22 Aug. 2007 |
| i0959a | ” | Hachinohe, Aomori, Japan | Local | 11 Aug. 2007 |
| i0981a | ” | Hachinohe, Aomori, Japan | Local | 19 Aug. 2007 |
| i1003a | ” | Kosaka Akita, Japan | Local | 1 Oct. 2007 |
| Wayamanashi i1104a | ” | Tono, Iwate, Japan | Local | 15 Aug. 2007 |
| i1115a | ” | Kuzumaki, Iwate, Japan | Local | 17 Sept. 2007 |
| Kosuia | P. pyrifolia (Burm.) Nak. | Japan | Cultivar | 26 Aug. 2007 |
| Nijisseikia | ” | ” | Cultivar | 11 Sept. 2007 |
| Niitakaa | ” | ” | Cultivar | 10 Oct. 2007 |
| Yakumod | ” | ” | Cultivar | 16 Sept. 2007 |
| Kaoria | ” | ” | Cultivar | 1 Nov. 2007 |
| Ya lia | P. bretschneideri Rehd. | China | Cultivar | 15 Nov. 2007 |
| La Francea | P. communis L. | France | Cultivar | 10 Nov. 2007 |
| Fujid | Malus × domestica Borkh. | Japan | Cultivar | 15 Nov. 2007 |
Materials maintained at FRC-KU, Food Resources Education and Research Center, Kobe University.
Wild grown materials harvested at Iwate.
Refer to Iketani et al. (2010), Iketani and Katayama (2012) for more information on two wild accessions.
Fruit samples were purchased from the market.
Preparation of odor concentrations
Fruit samples were harvested at full maturity. Iodine staining of starch has been used to judge maturity of fruit used in this study. For AEDA, two thousand grams of fruit without core and peduncle were homogenized with a blender. Three hundred grams of calcium chloride and 1 L of distilled water were added to inhibit enzyme activity before blending (Buttery et al. 1987). The suspension was centrifuged at 15000 rpm for 10 min at 4°C. The supernatant was passed through a glass column (20 mm × 300 mm) packed with 9 g of Tenax TA. The odor components in the supernatant absorbed by Tenax TA were desorbed slowly with purified diethyl ether (200 ml). The ether eluents containing butyl benzene as an internal standard were dehydrated with Na2SO4 overnight and concentrated in a water bath at 40°C. For PCA, odor concentrations from 24 accessions were prepared with same procedures at one-tenth scale of AEDA.
Identification of odor compounds by GC/MS
GC/MS analyses were carried out using a GC-2010 (Shimadzu, Japan) gas chromatograph coupled to a mass spectrometer (QP-2010, Shimadzu, Japan).
Gas chromatography was carried out using a Shimadzu GC-2010 gas chromatograph fitted with a fused silica capillary column DB-WAX; (60 m in length, 0.25 mm i.d. and 0.25 μm film thickness; J & W Scientific, USA). Helium at a flow rate of 0.8 ml/min was used as the carrier gas. The oven temperature was programmed from 40 to 200°C at the rate of 3°C/min. The temperature of the injection port was maintained at 210°C. Electron ionization mass spectra were obtained at an ionization voltage of 70 eV and a source temperature of 240°C. The MS compound peaks for each material were then integrated using Shimadzu GC/MS solutions software (version 2.21, Shimadzu, Japan). Volatile compounds were tentatively identified using computerized mass spectroscopic libraries (Wiley version 7 database) and the Kovats index (KI). In order to determine the KI of the compounds, a mixture of n-alkanes (C6-C21) was used (Kovats 1965). Further identification was based on comparison of mass spectra and retention times between the compounds detected and authentic compounds analyzed under the same conditions.
GC-O and AEDA
GC/O analyses were performed using a GC-17A (Shimadzu, Japan) gas chromatograph fitted with a fused silica capillary column DB-WAX equipped with Flame Ionization Detection (FID). GC/O analyses were carried out under the same conditions as the GC-MS analyses. GC/O involves the effluent being split into equal parts at the end of the column, each part being respectively conveyed to FID and a sniffing port. The peak areas were calculated using a Shimadzu Chromatopack C-R7A integrator. To quantify the odor-active compounds, standard curves for odor-active compounds were established using authentic 3 standards such as ethyl butanoate, trans-2-hexanal and ethyl hexanoate (i.e., 5 concentrations for 3 compounds). Mean recovery concentration and rate of standard chemicals concentrated by Tanax TA column extraction were measured. The accuracy of the standard curves was high as indicated by linear correlation coefficient (r), which ranged from 0.9955 to 1.0000. 20% butyl benzene (0.81 ppm) was spiked as the internal standard in each sample. The concentrations of each odor compound were calculated based on the ratio of peak areas to the internal standard as follows:
All experiments were performed twice, in duplicate for accessions.
Serial dilutions of the fruit odor concentrate with diethyl ether were assessed by GC/O, and FD factors of the odor-active compounds in pears were determined by AEDA. Data of GC/O by two panelists were used for AEDA. Two panelists evaluated odor description and flavour dilution factor for each peak of gas chromatograph. The odor concentrate was diluted sequentially at 1 : 1 ratio. Each dilution was analyzed until the odor could no longer be detected. FD factors were calculated based on the last dilution.
Principal component analysis
Eleven compounds were detected with GC and were tentatively identified by comparing retention indexes with those of internal standards and KI data. To confirm the GC data, 23 pears were tested twice. The concentrations of the 11 compounds were calculated by comparing the peak area ratio of each internal standard to the compound among 24 accessions (Table 3). Calculation of Principal Component Analysis (PCA) was performed with JMP software (Version 8.0, SAS Institute Inc.).
Table 3.
Concentration of 11 odor-active compounds in fruit extracts
| No. | Compound | KIa | Concentration (ppm)b ± standard error | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ‘Kosui’ | ‘Nijisseiki’ | ‘Niitaka’ | ‘Yakumo’ | ‘Kaori’ | ‘Ya li’ | ‘La France’ | |||
| 1 | ethyl 2-methypropanoate | 943 | 0.002 ± 0.001 | ndc | 0.002 ± 0.002 | ndc | 0.035 ± 0.002 | 0.054 ± 0.008 | ndc |
| 2 | methyl 2-methylbutanoate | 1008 | 0.016 ± 0.001 | <0.001 | <0.001 | ndc | 0.002 | 0.001 ± 0.001 | ndc |
| 3 | ethyl butanoate | 1033 | 0.013 ± 0.009 | 0.006 ± 0.003 | 0.039 ± 0.035 | 0.062 ± 0.021 | 4.190 ± 0.725 | 4.117 ± 0.482 | 0.140 ± 0.021 |
| 4 | ethyl 2-methylbutanoate | 1047 | 0.001 ± 0.001 | ndc | 0.001 ± 0.001 | ndc | 0.069 ± 0.011 | 0.126 ± 0.019 | ndc |
| 5 | butyl acetate | 1065 | 0.008 ± 0.001 | 0.011 ± 0.001 | 0.009 ± 0.001 | 0.006 ± 0.001 | 0.017 ± 0.002 | 0.120 ± 0.008 | 30.722 ± 3.660 |
| 6 | hexanal | 1072 | 0.217 ± 0.008 | 0.145 ± 0.024 | 0.228 ± 0.067 | 0.236 ± 0.059 | 0.406 ± 0.088 | 1.194 ± 0.126 | 0.360 ± 0.056 |
| 7 | ethyl pentanoate | 1127 | 0.003 | <0.001 | <0.001 | 0.003 ± 0.001 | 0.028 ± 0.001 | 0.027 ± 0.002 | 0.009 ± 0.001 |
| 8 | pentyl acetate | 1170 | ndc | ndc | ndc | ndc | <0.001 | 0.001 | 0.346 ± 0.057 |
| 9 | trans-2-hexenal | 1212 | 0.151 ± 0.014 | 0.025 ± 0.004 | 0.099 ± 0.024 | 0.038 ± 0.014 | 0.112 ± 0.022 | 0.170 ± 0.009 | 0.341 ± 0.073 |
| 10 | ethyl hexanoate | 1234 | 0.004 ± 0.002 | 0.002 | 0.007 ± 0.006 | 0.001 ± 0.001 | 0.165 ± 0.034 | 0.495 ± 0.090 | 0.002 ± 0.002 |
| 11 | hexyl acetate | 1273 | 0.025 ± 0.012 | ndc | ndc | ndc | 0.029 ± 0.002 | 0.034 ± 0.005 | 16.797 ± 3.826 |
|
| |||||||||
| No. | Concentration (ppm)b ± standard error | ||||||||
|
| |||||||||
| ‘Fuji’ | i0009 | i1302 | i01701 | ‘Sanenashi’ | i0187 | i0193 | i0218 | i0471 | |
|
| |||||||||
| 1 | 0.001 ± 0.001 | 0.113 ± 0.022 | 0.011 ± 0.003 | 0.150 ± 0.026 | 0.006 ± 0.003 | ndc | 0.029 ± 0.001 | 0.001 ± 0.001 | ndc |
| 2 | 0.001 ± 0.001 | 0.016 ± 0.004 | 0.003 | 0.034 ± 0.011 | 0.017 ± 0.003 | 0.008 | 0.008 ± 0.002 | 0.001 ± 0.001 | 0.007 |
| 3 | 0.174 ± 0.014 | 4.006 ± 0.623 | 0.989 ± 0.357 | 4.015 ± 0.189 | 1.112 ± 0.359 | 0.029 ± 0.004 | 1.862 ± 0.763 | 0.119 ± 0.012 | 0.256 ± 0.125 |
| 4 | 0.001 ± 0.001 | 0.472 ± 0.095 | 0.043 ± 0.008 | 0.601 ± 0.124 | 0.025 ± 0.013 | 0.005 ± 0.002 | 0.179 ± 0.010 | 0.001 ± 0.001 | 0.033 ± 0.021 |
| 5 | 0.215 ± 0.006 | 0.287 ± 0.031 | 0.248 ± 0.029 | 0.134 ± 0.014 | 1.621 ± 0.408 | 0.033 ± 0.002 | 0.017 ± 0.009 | 0.055 ± 0.025 | 0.028 ± 0.014 |
| 6 | 0.014 ± 0.001 | 0.256 ± 0.009 | 0.397 ± 0.034 | 0.817 ± 0.091 | 0.955 ± 0.157 | 2.195 ± 0.230 | 1.149 ± 0.338 | 0.871 ± 0.114 | 1.440 ± 0.188 |
| 7 | 0.011 ± 0.001 | 0.033 ± 0.002 | 0.038 ± 0.011 | 0.039 ± 0.001 | 0.019 ± 0.003 | 0.003 | 0.004 ± 0.002 | 0.022 ± 0.002 | ndc |
| 8 | 0.007 ± 0.001 | 0.008 ± 0.001 | 0.004 ± 0.001 | 0.005 ± 0.002 | 0.012 ± 0.005 | 0.001 ± 0.001 | <0.001 | ndc | ndc |
| 9 | 0.468 ± 0.043 | 0.718 ± 0.089 | 0.803 ± 0.100 | 2.126 ± 0.011 | 2.171 ± 0.383 | 2.846 ± 0.217 | 1.669 ± 0.047 | 1.052 ± 0.505 | 0.905 ± 0.085 |
| 10 | 0.003 ± 0.002 | 2.730 ± 0.521 | 0.606 ± 0.224 | 1.673 ± 0.203 | 0.136 ± 0.071 | 0.008 ± 0.005 | 0.262 ± 0.052 | 0.002 ± 0.002 | 0.035 ± 0.025 |
| 11 | 0.038 ± 0.001 | 0.254 ± 0.019 | 0.078 ± 0.027 | 0.263 ± 0.021 | 0.177 ± 0.052 | 0.003 ± 0.003 | 0.046 ± 0.020 | 0.011 ± 0.006 | 0.026 ± 0.003 |
|
| |||||||||
| No. | Concentration (ppm)b ± standard error | ||||||||
|
| |||||||||
| i0605 | i0830 | i0868 | i0959 | i0981 | i1003 | i1104 | i1115 | ||
|
| |||||||||
| 1 | 0.063 ± 0.010 | ndc | 0.031 ± 0.005 | 0.004 | 0.021 | 0.106 ± 0.007 | 0.022 | 0.007 ± 0.001 | |
| 2 | 0.022 ± 0.002 | 0.002 ± 0.001 | 0.039 ± 0.008 | ndc | 0.001 ± 0.001 | 0.018 ± 0.003 | 0.004 | ndc | |
| 3 | 3.660 ± 0.617 | 0.341 ± 0.066 | 1.332 ± 0.015 | 0.198 ± 0.038 | 0.921 ± 0.064 | 3.782 ± 0.583 | 0.568 ± 0.102 | 0.740 ± 0.007 | |
| 4 | 0.477 ± 0.058 | 0.027 ± 0.008 | 0.215 ± 0.009 | 0.012 ± 0.001 | 0.089 ± 0.005 | 0.259 ± 0.065 | 0.065 ± 0.011 | 0.024 ± 0.005 | |
| 5 | 3.171 ± 0.644 | 1.840 ± 0.022 | 0.323 ± 0.065 | 0.133 ± 0.030 | 0.949 ± 0.019 | 3.480 ± 0.413 | 0.015 ± 0.001 | 0.631 ± 0.055 | |
| 6 | 0.001 | 0.066 ± 0.021 | 0.201 ± 0.033 | 0.342 ± 0.076 | 0.787 ± 0.315 | 1.041 ± 0.576 | 1.603 ± 0.432 | 1.599 ± 0.081 | |
| 7 | ndc | ndc | 0.028 ± 0.003 | 0.001 ± 0.001 | 0.021 ± 0.001 | 0.043 ± 0.006 | 0.003 ± 0.001 | 0.008 ± 0.001 | |
| 8 | 0.034 ± 0.005 | 0.020 ± 0.002 | 0.009 ± 0.001 | 0.002 ± 0.001 | 0.007 ± 0.006 | 0.079 ± 0.008 | ndc | 0.006 ± 0.001 | |
| 9 | 0.216 ± 0.096 | 0.664 ± 0.089 | 1.791 ± 0.395 | 0.560 ± 0.178 | 0.950 ± 0.067 | 1.795 ± 0.701 | 2.474 ± 0.752 | 1.870 ± 0.011 | |
| 10 | 3.105 ± 0.543 | 0.087 ± 0.023 | 0.545 ± 0.019 | 0.064 ± 0.011 | 0.395 ± 0.080 | 1.304 ± 0.220 | 0.124 ± 0.003 | 0.229 ± 0.015 | |
| 11 | 0.982 ± 0.143 | 0.723 ± 0.087 | 0.107 ± 0.014 | 0.026 ± 0.007 | 0.129 ± 0.005 | 3.292 ± 1.376 | 0.004 ± 0.001 | 0.253 ± 0.040 | |
KI means Kovats Index calculated for the DB-Wax capillary column (60 m × 0.25 mm i.d.).
Mean values (±SE) obtained from experiments performed twice in duplicate were calculated.
nd: not detected due to too low peak or no peak.
Results
Identification of odor-active compounds
Odor descriptions from sensory analysis were fresh and fruity for ‘Sanenashi’, sweet and fresh for i0830, sweet for ‘Kosui’ and fruity for ‘La France’. The yields of odor concentrates from 2000 g fruit were 72.4 mg from ‘Sanenashi’, 61.3 mg from i0830, 27.5 mg from ‘Kosui’ and 45.8 mg from ‘La France’. The yields of odor concentrates from ‘Sanenashi’ and i0830 were higher than those of ‘Kosui’ and ‘La France’. The number of odors detected with GC/O was 116, 112, 68 and 58 compounds in ‘Sanenashi’, i0830, ‘Kosui’ and ‘La France’, respectively. The number of odor compounds in ‘Sanenashi’ and i0830 was almost twice that in ‘Kosui’ and ‘La France’. Twenty-two, 29, 9 and 15 odor compounds represented with GC/O in the concentrates of ‘Sanenashi’, i0830 ‘Kosui’ and ‘La France’, respectively, was identified by GC/MS. The KI, odor descriptions and FD factors by AEDA of the odor compounds in ‘Sanenashi’ and i0830 of Iwateyamanashi, ‘Kosui’ and ‘La France’ are shown in Table 2. The number of odor-active compounds exhibiting a high FD factor (FD ≥32) in ‘Sanenashi’, i0830 and ‘La France’ (18, 20 and 19 odors) was about three times higher than that of ‘Kosui’ (6 odors). In ‘Sanenashi’, an unknown compound with the odor descriptions of medicinal, floral and green had the highest FD factor (8192) at a KI of 2170 (Table 2). Nine odor-active compounds were identified in the odor concentrations of ‘Sanenashi’ in the range KI 943–1234 (FD ≥32). These were ethyl 2-methylpropanoate (fresh and apple-like), methyl 2-methylbutanoate (fruity, fresh and apple-like), ethyl butanoate (fruity and sweet), ethyl 2-methylbutanoate (fresh, fruity and pineapple-like), hexanal (green), ethyl pentanoate (fruity), cis 3-hexenal (green), trans 2-hexanal (fresh and green) and ethyl hexanoate (fruity and sweet). The other 9 odor-active compounds in ‘Sanenashi’, cis 3-hexenol (green and cucumber-like), 2-phenylethanol (rose-like) and 7 unknown compounds (FD ≥32), were detected in the range KI 1300–2170 (Table 2). In i0830, twenty odor-active compounds in the FD factor range of 32–4096 were detected. An unknown odor-active compound exhibited the highest FD (4096). This was also the highest FD (8192) in ‘Sanenashi’ at KI 2170. Six odor-active compounds were detected in the range KI 1033–1300 (FD ≥32): ethyl butanoate (fruity and sweet), ethyl 2-methylbutanoate (fresh, fruity and pineapple-like), cis 3-hexenal (green), trans 2-hexanal (fresh and green), hexyl acetate (fruity) and a unknown compound (citrus-like). Six odor-active compounds were found in the range KI 1307–1527 (FD ≥32): acetic acid (sour), 3-(methylthio)-1-propanal (potato-like) and 4 unknown compounds. Eight odor-active compounds, 2-phenylethanol (rose-like) and 7 unknown compounds (barley-like, alcohol, citrus-like, apricot-likes, medicinal, floral, green, fruity and metallic), were detected in the range KI 1820–2175. There were more odor-active compounds detected in i0830 than in ‘Sanenashi’ (Table 2).
Table 2.
The comparison of FD factors for odor-active compounds (FD factor ≥32) among four pears
| No. | KIa | Compound | Odor descriptionc | FD factor | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ‘Sanenashi’ | i0830 | ‘Kosui’ | ‘La France’ | ||||
| 1 | 943 | Ethyl 2-methylpropanoate | fresh, apple-like | 32 | 16 | 1 | |
| 2 | 1008 | Methyl 2-methylbutanoate | fruity, fresh, apple-like | 32 | 1 | 1 | |
| 3 | 1033 | Ethyl butanoate | fruity, sweet | 256 | 32 | 64 | |
| 4 | 1047 | Ethyl 2-methylbutanoate | fresh, fruity, pineapple-like | 512 | 512 | 256 | 1 |
| 5 | 1065 | Butyl acetate | fruity | 8 | 32 | ||
| 6 | 1072 | Hexanal | green | 512 | 8 | 16 | 64 |
| 7 | 1127 | Ethyl pentanoate | fruity | 32 | 4 | 4 | |
| 8 | 1130 | Cis-3-hexenalb | green | 2048 | 32 | 16 | 2 |
| 9 | 1170 | Pentyl acetate | fruity | 1 | 1 | 32 | |
| 10 | 1212 | Trans-2-hexenal | fresh, green | 256 | 64 | 32 | |
| 11 | 1234 | Ethyl hexanoate | fruity, sweet | 128 | 1 | 4 | |
| 12 | 1273 | Hexyl acetate | fruity | 2 | 32 | 32 | |
| 13 | 1296 | citrus-like | 2 | 64 | 16 | 16 | |
| 14 | 1300 | metallic | 32 | 1 | 8 | ||
| 15 | 1307 | apple-like, fresh | 32 | ||||
| 16 | 1350 | floral | 2 | 32 | |||
| 17 | 1371 | burnt metal-like | 128 | 1 | 2 | ||
| 18 | 1383 | Cis-3-hexenolb | green, cucumber-like | 128 | 1 | 1 | |
| 19 | 1439 | Acetic acidb | sour | 8 | 32 | 8 | 32 |
| 20 | 1462 | 3-(methylthio)-1-propanalb | potato-like | 8 | 32 | 128 | 64 |
| 21 | 1485 | lemony | 32 | 1 | |||
| 22 | 1527 | fruity, apple-like | 32 | 4 | 2 | ||
| 23 | 1590 | green banana-like, sweet | 8 | 1 | 256 | 32 | |
| 24 | 1629 | citrus-like, unpleasant | 32 | 4 | 256 | ||
| 25 | 1736 | sweet | 32 | ||||
| 26 | 1755 | fruity, banana-like | 1 | 32 | |||
| 27 | 1820 | barley-like | 32 | 32 | 1 | 32 | |
| 28 | 1830 | tea-like, alcohol-like | 1024 | 32 | 1 | 4 | |
| 29 | 1925 | 2-phenylethanol | rose-like | 64 | 128 | 1 | |
| 30 | 1930 | citrus-like, green | 2 | 64 | 32 | ||
| 31 | 1977 | European pear-like | 8 | 32 | |||
| 32 | 1993 | metallic | 1 | 64 | 32 | ||
| 33 | 2010 | fruity, fresh | 1 | 1 | 32 | ||
| 34 | 2160 | Japanese apricot-like, fruity | 512 | 64 | 8 | 1 | |
| 35 | 2170 | medicinal, floral, green | 8192 | 4096 | 64 | 64 | |
| 36 | 2175 | fruity | 2 | 32 | |||
| 37 | 2225 | medicinal | 1 | 32 | |||
| 38 | 2260 | fresh cream-like, vanilla-like | 1 | 1 | 32 | ||
| 39 | 2560 | vanilla-like | 8 | 8 | 8 | 32 | |
KI means Kovats Index calculated for the DB-Wax capillary column (60 m × 0.25 mm i.d.).
Tentatively identified.
Odor descriptions obtained from GC/O.
Six and 19 odor-active compounds in the FD range of 32–256 were detected in ‘Kosui’ and ‘La France’ respectively (Table 2). Six odor-active compounds, ethyl butanoate, ethyl 2-methylbutanoate, 3- methylthio propanal (potato-like) and three unknown compounds (banana-like, fruity and medicinal) were found in the range KI 1033–2170 in ‘Kosui’. The FD factor of an unknown compound at KI 1590 was especially high in ‘Kosui’ (green banana-like and sweet). In ‘La France’, ten odor-active compounds were detected in the range KI 1065–1736: butyl acetate (fruity), hexanal (green), pentyl acetate (fruity), trans 2-hexanal (fresh and green), hexyl acetate (fruity), acetic acid (sour), 3-(methylthio)-propanal (potato-like) and 3 unknown compounds (banana-like, citrus-like and sweet). Twelve unknown compounds (barley, citrus, European pear-like, vanilla-likes, metallic, fruity, medicinal, floral and green) were detected in the range KI 1820–2560. Acetate esters such as butyl acetate, pentyl acetate and hexyl acetate were found to be the major odor-active components of ‘La France’ in this study.
Comparison of FD factors among 4 pears
Ethyl esters (ethyl butanoate and ethyl 2-methylbutanoate) which contribute to sweet, apple-like, fruity odors were detected at high FD factors in ‘Sanenashi’, i0830 and ‘Kosui’. Hexyl acetate (fruity), acetic acid (sour), 3-(methylthio)-1-propanal (potato-like) and 8 unknown compounds (FD ≥32) at KI 1296, 1307, 1350, 1485, 1527, 1930, 1993 and 2175 were detected at high FD factors in i0830, but at a lower level in ‘Sanenashi’. The FD factors of 2-phenylethanol (rose-like) and 2 unknown compounds (tea-like, alcohol, apricot-like and fruity) at KI 1830 and 2160 were particularly high in both ‘Sanenashi’ and i0830. In a comparison between the FD factors of ‘Sanenashi’ and i0830, 16 odors (ethyl 2-methylpropanoate, methyl-2-methylbutanoate, ethyl butanoate, butyl acetate, hexanal, ethyl pentanoate, cis-3-hexanal, trans-2-hexenal, ethyl hexanoate, cis-3-hexanol and 6 unknown compounds at KI 1300, 1371, 1629, 1830, 2160 and 2170) were higher in ‘Sanenashi’ than in i0830. The FD factors of ethyl esters such as ethyl butanoate, ethyl hexanoate and aldehydes such as hexanal, cis-3-hexanal and alcohol such as cis-3-hexanol were especially high in ‘Sanenashi’. Trans-2-hexanal, hexyl acetate, acetic acid, 3-(methylthio)-1-propanal and 4 unknown compounds (KI 1820KI 1930KI 1993 and 2170) were detected at high FD factors (FD ≥32) in i0830 and ‘La France’.
Eleven volatile compounds from Iwateyamanashi used for PCA
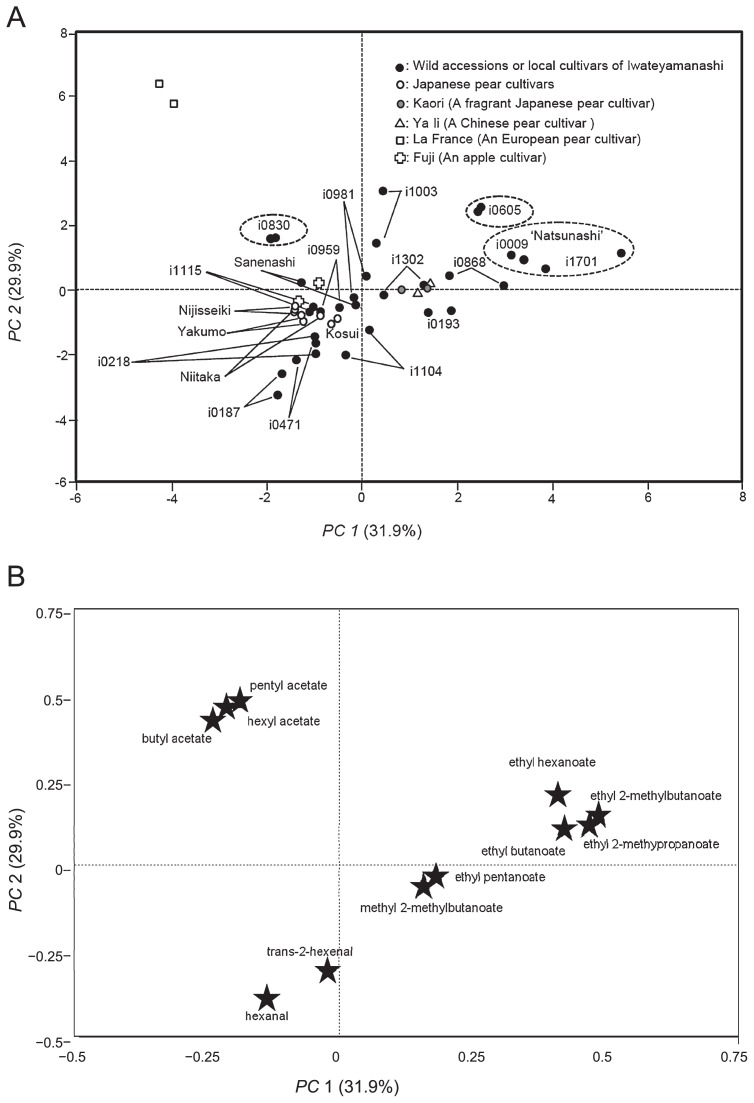
In order to demonstrate the relationships among 24 accessions based on fruit odor, PCA was carried out using the value of 11 odor-active compounds (FD ≥32), ethyl-2-methylpropanoate, methyl 2-methylbutanoate, ethyl butanoate, ethyl 2-methylbutanoate, hexanal, ethyl pentanoate, trans-2-hexanal and ethyl hexanoate from ‘Sanenashi’ and the 3 compounds, hexyl acetate, butyl acetate and pentyl acetate (FD ≥32) from i0830 and ‘La France’ estimated by AEDA analysis, were selected for PCA (Fig. 1). Eleven compounds were detected with GC and were tentatively identified by comparing retention indexes with those of internal standards and KI. GC analysis was basically done for two times for each accession. It was small as a number of repetition but short harvest time made it difficult to increase the repeat number because it was important to use fresh materials for analysis just after harvesting. Exceptionally some accessions, e.g., ‘Sanenashi’ grown wild were analyzed only one time because of their biennial bearing (Table 1). The concentrations of the 11 compounds were calculated by comparing the peak area ratio of each internal standard to the compound among 24 accessions (Table 3). High concentrations were measured for ethyl butanoate, butyl acetate, hexanal, trans-2-hexanal, ethyl hexanoate and hexyl acetate. Ethyl butanoate was contained in ‘Kaori’, ‘Ya li’, i0009, i1701, ‘Sanenashi’, i0193, i0605, i0868 and i1003 at high concentration of 1.1 to 4.2 ppm. Butyl acetate was contained in ‘La France’ at high concentration of 30.7 ppm. Hexanal and tans-2-hexanal were highest in i0187 at concentration of 2.2 and 2.8 ppm. Ethyl hexanoate was contained in i0009 and i0605 at high concentration of 2.7 and 3.1 ppm. Hexyl acetate was highest in ‘La France’ at concentration of 16.8 ppm (Table 3).
In order to demonstrate the relationships between 24 accessions based on fruit odors, PCA was carried out using the values of the 11 volatile compounds described above. About 61.8% variation was exhibited by the first and the second components. This is shown in Fig. 2, where the 24 accessions are projected on the first and second axis. Accessions with the ethyl and methyl esters, ethyl hexanoate, ethyl-2-methylpropanoate, ethyl butanoate and ethyl 2-methylbutanoate are located on the right upper side. i0605, i0868 and local cultivars ‘Natsunashi’ such as i1701 and i0009 are on the right upper side. i1701, in particular, is on the far right side. Ethyl pentanoate and methyl-2-methylbutanoate are plotted on the right lower side. Local cultivar ‘Wayamanashi’ (i1104-1) and i0193 are on the right lower side. ‘Ya li’, ‘Kaori’ and i1302 (homonym of ‘Natsunashi’) exhibited both ethyl and methyl esters and are in the second quadrant. In contrast, all the Japanese pear cultivars, with the exception of ‘Kaori’, with aldehydes such as hexanal and trans-2-hexenal are located on the left lower side. i0187, i0471, i0218, i1115, i0959, i1104-2 and i981-2 of Iwateyamanashi all map to the left lower side. ‘La France’, which has acetate esters such as pentyl, butyl and hexyl acetates, maps to the uppermost left. The wild accession i0830, ‘Sanenashi’ of Iwateyamanashi and an apple cultivar ‘Fuji’ also appear on the upper left side. i1003 and i0605 with both acetate and ethyl esters are located in the right upper side.
Fig. 2.
Principal component scores (A) and loading plot (B) for principal component 1 and 2. ● Local cultivars and wild accessions of Iwateyamanashi, ○ Japanese pear cultivars,
 ‘Kaori’, △ ‘Ya li’, □ ‘La France’,
‘Kaori’, △ ‘Ya li’, □ ‘La France’,
 ‘Fuji’ and ★ Eleven odor-active compounds. The dashed circles indicate two accessions of ‘Natsunashi’ and two wild accessions in Iwateyamanashi.
‘Fuji’ and ★ Eleven odor-active compounds. The dashed circles indicate two accessions of ‘Natsunashi’ and two wild accessions in Iwateyamanashi.
Discussion
Odor-active compounds detected from ‘Sanenashi’ and i0830
The numbers of odor-active compounds (116 and 112) detected by GC/O in a local cultivar ‘Sanenashi’ and a wild accession i0830 of Iwateyamanashi were almost twice those (68 and 58) in ‘Kosui’ and ‘La France’. ‘Sanenashi’ and i0830 contained various active compounds. The majority of these were C6-compounds. By comparing FD factors, C6-aldehydes such as hexanal, cis-3-hexenal and trans-2-hexenal and alcohol such as cis-3-hexenol are thought to be the major odor-active compounds in ‘Sanenashi’, but are minor components in i0830. These C6-aldehydes could contribute to the fresh odor of ‘Sanenashi’. Furthermore, an unknown odor-active compound (KI 2170) exhibiting the highest FDs of 8192 for ‘Sanenashi’ and 4096 for i0830 are thought to contribute mainly to fresh and green odor notes. Further experiments are needed to identify unknown odor-active compounds. Methyl 2-methylbutanoate and ethyl esters such as ethyl 2-methylpropanoate, ethyl butanoate, ethyl-2-methylbutanoate, ethyl pentanoate and ethyl hexanoate were the major esters in ‘Sanenashi’ and i0830 in the present study. Especially ethyl butanoate, ethyl-2-methylbutanoate and ethyl hexanoate for ‘Sanenashi’ (FD ≥128), ethyl-2-methylbutanoate and ethyl butanoate for i0830 (FD ≥32), in particular, had high FD values (Table 2). Though ethyl hexanoate was identified in 3 Japanese pear cultivars as one of major odor-active components (Horvat et al. 1992), the FD value of that was found to be small in ‘Kosui’ in this study. Fresh and fruity odors of ‘Sanenashi’ and sweet and fresh odors of i0830 might be caused by these ethyl and methyl esters. Butyl acetate, pentyl acetate and hexyl acetate, identified as important odor compounds in European pears, were detected in ‘Sanenashi’, i0830 and ‘La France’, while acetate esters in Asian pears such as 4 Japanese pear cultivars, 2 Chinese sand and white pear cultivars ‘Seuri’ and ‘Ya li’ were reported as minor components in a previous studies (Horvat et al. 1992, Imayoshi et al. 1995, Shiota 1990, Suwanagul and Richardson 1998, Takeoka et al. 1992). Hexyl acetate, in particular, exhibited a high FD value (FD 32) in i0830 (Table 2). These acetate esters could contribute to the fresh and fruity odors of ‘Sanenashi’ and sweet and fresh odors of i0830. Butyl acetate and hexyl acetate might be important odor-active compounds in Iwateyamanashi (Tables 2, 3). 2-phenylethanol (rose-like) appeared to be an important odor-active compound (KI 1925) in ‘Sanenashi’ and i0830. Though 2-phenylethanol has been found in small amounts in the European pear ‘Packham’s Triumph’ (Chervin et al. 2000), to date, this compound has not been detected in Japanese pears. 2-phenylethanol with a rose-like odor profile has been described as a typical odor-active compound in Rose (Clark 1990). The sweet and roselike odors of ‘Sanenashi’ and i0830 might be caused by 2-phenylethanol. This alcohol could also be present as an odor-active compound in Iwateyamanashi. Furthermore, whether ‘Sanenashi’ odor can reconstitute by compounds exhibiting high FD value (FD ≥32) with AEDA, sensory evaluations of a model mixture of ‘Sanenashi’ were performed according to Quantitative Descriptive Analysis (QDA) described previously by Shimoda et al. (1989a, 1989b), Stone et al. (1974) and Stone and Sidel (1993). From the result of QDA, ‘Sanenashi’ odor was reconstituted nearly by 8 compounds such as ethyl 2-methylpropanoate, methyl 2-methylbutanoate, ethyl butanoate, ethyl 2-methylbutanoate, hexanal, ethyl pentanoate, trans-2-hexenal and ethyl hexanoate (data not shown).
Variable and rich odors of Iwateyamanashi
The concentrations of the 11 odor-active compounds varied considerably between the 16 accessions of Iwateyamanashi. These results reflect the wide range of genetic diversity in Iwateyamanashi which has already been revealed by morphological and molecular analyses (Katayama and Uematsu 2006, Katayama et al. 2007, Matsumura et al. 2011). Local cultivars and wild accessions in Iwateyamanashi were located in all quadrants of the PCA plot. These results are indicative of the wide range of odor diversity in Iwateyamanashi. In contrast, all Japanese pear cultivars, with the exception of ‘Kaori’, harboring aldehydes such as hexanal and trans-2-hexenal were grouped on the left lower side. These results are thought to reflect the low genetic diversity of odor compounds in Japanese pear cultivars. i1701 and i0009 (homonyms of ‘Natsunashi’), i0605 and i0868 which contain ethyl and methyl esters are located on the right upper side. In particular, an early matured local cultivar ‘Natsunashi’ (i1701 and i0009) exhibited high concentrations of ethyl and methyl esters in comparison with the cultivars ‘Ya li’ and ‘Kaori’ with sweet odors. ‘Natsunashi’ might be useful as a donor of sweet strong odors for Japanese pear breeding in future. i1003, located on middle upper side, is supposed to possess well balanced odor-active compounds such as ethyl, methyl and acetate esters. Two accessions of i0605 locating on right upper side and i0830 on left upper side had also high ester concentration. These two accessions were revealed to be wild Iwateyamanashi trees by means of the populational structure analysis using SSR markers (Iketani et al. 2010), but these were plotted on different quadrants in PCA. This result suggest the wide range of odor diversity in wild Iwateyamanashi trees. Three accessions such as i1003, i0605 and i0830 are also available as donors of fragrance for pear breeding. By surveying odor-active compounds in Iwateyamanashi, this investigation, has revealed the presence of variable and rich odors, which modern Japanese pear cultivars lack. Iwateyamanashi represents a valuable genetic resource as donor of odors for Japanese pear breeding.
Acknowledgements
Thanks are due to Mr. S. Kakehi, Mr. K. Masaki, Mr. T. Tanigawa and Ms Y. Watanabe in Food Resources Research and Education Center, Kobe University, for maintenance of plant materials. The authors are grateful to Dr. M. Ohata for helpful suggestions and arrangements for the experiments. Sincere appreciation is expressed to Dr. Anne Edwards, John Innes Centre, UK, for her English correction and useful suggestions. This work was supported in part by Grant-in-Aids (No. 19580031) for Scientific Research from the Ministry of Education, Science and Culture, Japan and by a General Research Grant from the NIAS Gene Bank.
Literature Cited
- Asami, Y. (1921) Nipponnashi no shurui. Nippon Engei Zasshi 33: 1–11 [Google Scholar]
- Buttery, R.G., Teranishi, R., and Ling, L.C. (1987) Fresh tomato aroma volatiles: a quantitative study. J. Agric. Food Chem. 35: 540–544 [Google Scholar]
- Chen, J.L., Yan, S., Feng, Z., Xiao, L., and Hu, X.S. (2006) Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bretschneideri Reld) during storage. Food Chem. 97: 248–255 [DOI] [PubMed] [Google Scholar]
- Chervin, C., Speirs, J., Loveys, B., and Patterson, B.D. (2000) Influence of low oxygen storage on aroma compounds of whole pears and crushed pear flesh. Postharv. Biol. Tech. 19: 279–285 [Google Scholar]
- Clark, G.S. (1990) Phenethyl alcohol. Perfum Flavor 15: 37–44 [Google Scholar]
- Horvat, R.J., Senter, S.D., and Chapman, G.W.Jr. (1992) Volatiles of ripe asian pears (Pyrus serotina Rehder). J. Essent. Oil Res. 4: 645–646 [Google Scholar]
- Iketani, H., Yamamoto, T., Katayama, H., Uematsu, C., Mase, N., and Sato, Y. (2010) Introgression between native and prehistorically naturalized (archaeophytic) wild pear (Pyrus spp.) populations in Northern Tohoku, Northeast Japan. Conserv. Genet. 11: 115–126 [Google Scholar]
- Iketani, H., and Katayama, H. (2012) Introgression and long-term naturalization of archaeophytes into native plants underestimated risk of hybrids. In: Povilitis, T. (ed.) Topics in Conservation Biology, In-Tech Educational and Publishing, pp. 43–56 [Google Scholar]
- Imayoshi, Y., Yukawa, C., Iwabuchi, H., and Baser, K.H.C. (1995) Volatile constituents of Chinese pear ‘Yali’. Proc. 13th Int. Congress of Flavours, Fragrances and Essential Oils, Turkey, 2: 15–19 [Google Scholar]
- Kajiura, I., and Sato, Y. (1990) Recent progress in Japanese pear (Pyrus pyrifolia Nakai) breeding, and descriptions of cultivars based on literature review. Bull. Fruit Tree Res. Sta. Extra No.1, 1–329 [Google Scholar]
- Katayama, H., and Uematsu, C. (2006) Pear (Pyrus species) genetic resources in Iwate, Japan. Genet. Res. Crop Evol. 53: 483–498 [Google Scholar]
- Katayama, H., Adachi, S., Yamamoto, T., and Uematsu, C. (2007) A wide range of genetic diversity in pear (Pyrus ussuriensis var. aromatica) genetic resources from Iwate, Japan revealed by SSR and chloroplast DNA markers. Genet. Res. Crop Evol. 54: 1573–1585 [Google Scholar]
- Kikuchi, A. (1946) Classification of the strains and varieties of the Chinese pear. Studies from the Inst. Hort., Kyoto Imper. Univ. 3: 1–11 [Google Scholar]
- Kovats, E. (1965) Gas chromatographic characterization of organic substances in the Retention Index System. Adv. Chromatog. 1: 229–247 [Google Scholar]
- Kotobuki, K. (2000) Nashi Hiratsuka 16 gou, a mid-maturing smooth-skin Japanese pear (Pyrus pyrifolia Nakai var. culta Nakai). Bull. Natl. Inst. Fruit Tree Sci. 34: 105–109 [Google Scholar]
- Matsumura, Y., Kakehi, S., Masaki, K., Miyake, M., Uematsu, C., and Katayama, H. (2011) Pear (Pyrus spp.) genetic resources from northern Japan: evaluation of threatened landraces for morphological and agronomical traits. Acta Hort. 918: 971–982 [Google Scholar]
- Ministry of Agriculture, Forestry and Fisheries, Japan (2011) The Statistical Year Data. Statistic department [Google Scholar]
- Morton, I.D., and MacLeod, A. (1990) J. Food flavours part C. In the flavour of fruits. Elsevier science publishers: Amsterdam, The Netherlands [Google Scholar]
- Nakai, T. (1919) Notulae ad plantas Japoniae et Koreae XXI. Bot. Mag. (Tokyo). 33: 193–216 [Google Scholar]
- Rapparini, F., and Predieri, S. (2002) Volatile constituents of ‘Harrow Sweet’ pears by dynamic headspace technique. Acta Hort. 596: 811–816 [Google Scholar]
- Rapparini, F., and Predieri, S. (2003) Pear fruit volatiles. Hort. Rev. 28: 237–324 [Google Scholar]
- Shimoda, M., Sasaki, H., Doi, Y., Kameda, W., and Osajima, Y. (1989a) Characterization of abstract terms for odor-description of food products. J. Jpn. Soc. Food Sci. Technol. 36: 7–16 [Google Scholar]
- Shimoda, M., Sasaki, H., Doi, Y., Kameda, W., and Osajima, Y. (1989b) Characterization of concrete terms for odor-description of food products. J. Jpn. Soc. Food Sci. Technol. 36: 17–25 [Google Scholar]
- Shiota, H. (1990) Changes in the volatile composition of La France pear during maturation. J. Sci. Food Agric. 52: 421–429 [Google Scholar]
- Stone, H., Sidel, J.L., Oliver, S., Woolsley, A., and Singleton, R.C. (1974) Sensory evaluation by qualitative descriptive analysis. Food Technol. 28: 24–32 [Google Scholar]
- Stone, H., and Sidel, J.L. (1993) Sensory evaluation practices, (2nd ed.), Academic press, inc, San Diego, CA, USA [Google Scholar]
- Suwanagul, A., and Richardson, D.G. (1998) Identification of headspace volatile compounds from different pear (Pyrus communis L.) varieties. Acta Hort. 475: 605–623 [Google Scholar]
- Takeoka, G.R., Buttery, R.G., and Flath, R.A. (1992) Volatile constituents of Asian pear (Pyrus serotina). J. Agric. Food Chem. 40: 1925–1929 [Google Scholar]
- Yamamoto, Y. (1997) Volatile constituents of pears. Koryo 196: 99–106 [Google Scholar]