Summary
The diagnosis of Bonnet-Dechaume-Blanc or Wyburn-Mason syndrome encompasses a spectrum of phenotypic expression. Features of the syndrome as originally described, and common to all, include arteriovenous malformations of the brain and orbit (with retinal and/or retrobulbar lesions). A portion of these patients manifest the complete expression of the disease with additional high-flow arteriovenous malformations of the maxillofacial or mandibular regions. These present the distinct and additional risks of life-threatening epistaxis or gingival haemorrhage. We suggest new diagnostic criteria for the syndrome. Applying insights from modern developmental biology to our series of 15 patients (the largest to date), together with a review of the literature`, we have recognised metameric patterns of involvement in what we believe to be a disease of the neural crest or adjacent cephalic mesoderm. This allows us to propose a new rational classification reflecting the putative, underlying disorder and to suggest a new name: Cerebrofacial Arteriovenous Metameric Syndrome (CAMS).
Key words: Bonnet-Dechaume-Blanc syndrome, Wyburn-Mason syndrome, cerebral arteriovenous malformations-multifocal, retinal vascular lesions, craniofacial vascular malformations, phakomatoses, metameric cerebral lesions, neural crest disease
Introduction
The condition of retinal arteriovenous malformation (AVM) was originally described by Magnus1 in 1874 and was for long regarded as a mere ophthalmological curiosity. Yates and Payne2 in 1932 described a patient with retinal and cerebral AVMs, but with only a single patient could not identify a syndrome. An association between arteriovenous malformations of the face, retina, and brain was first recognised by Bonnet, Dechaume and Blanc3, who reported 2 cases in Lyon in 1937. Six years later, at Queen Square in London, Wyburn-Mason4 reviewed all cases previously described and added nine further examples in a long and detailed study. The association of retinal, facial and cerebral vascular malformations became known as Bonnet-Dechaume-Blanc syndrome in France and continental Europe, and Wyburn-Mason syndrome in the English literature. The degree of expression of the syndromes' components varies, both clinically and morphologically. Thus the most fully expressed cases with maxillofacial AVMs, in addition to the orbital and intracranial lesions, are susceptible to life-threatening epistaxis or gingival bleeding in addition to the risks of blindness or cerebral haemorrhage. Some confusion has existed regarding the use of the two names; the term Bonnet-Dechaume-Blanc sometimes being preferred for the more extensive end of the disease spectrum with high-flow maxillofacial AVMs. Careful reading of the original articles3,4 however confirms that both were referring to the same condition. We thus use the two eponymous names of the syndrome interchangeably.
While remaining a very rare condition, its interest transcends its obscurity for it offers insight into the development of AVMs in general and further, into the underlying metameric structure of the developing vasculature of the brain and face, and the metameric diseases (including the better known Sturge-Weber syndrome) thereof. Few descriptions of the syndrome exist with the benefit of modern imaging techniques. Moreover, other than the original descriptions (of nine and two cases) and two other reports of three and two cases, all the remaining descriptions are based on single case reports. In reviewing our series of 15 cases, the largest ever published, we derived new diagnostic criteria as an aid to diagnosis. We were able to discern underlying patterns of involvement reflecting the metameric nature of the cerebrofacial structures and suggesting a disorder of neural crest development. We then compared our findings with previously published cases, which allowed us to propose a new rational classification of the syndrome. In the light of insights gained from developmental biology into the metameric organisation of the central nervous system, facial skeleton and their vasculature, we intimate how the various craniofacial syndromes could be related, how they might develop and further, how they relate to metameric disease of the spinal cord.
It is not our purpose here to address in detail the issues of natural history or therapeutic management.
Patients and Methods
Fourteen of these 15 patients form part of a large cohort of over 1500 patients with brain AVMs referred for assessment or treatment at Bicetre hospital since 1982. This is not a population-based cohort, with patients coming from many parts of Europe, Africa and Asia; indeed no population-based study would be likely to encounter so many patients with Wyburn-Mason syndrome. In the series of Willinsky et Al5, for example, of 213 patients with multifocal vascular malformations there was but a single case, while in the Scottish Intracranial Vascular Malformations Study, a true population based epidemiological study, in the first two years, only one case was encountered among 100 brain AVMs (unpublished data).
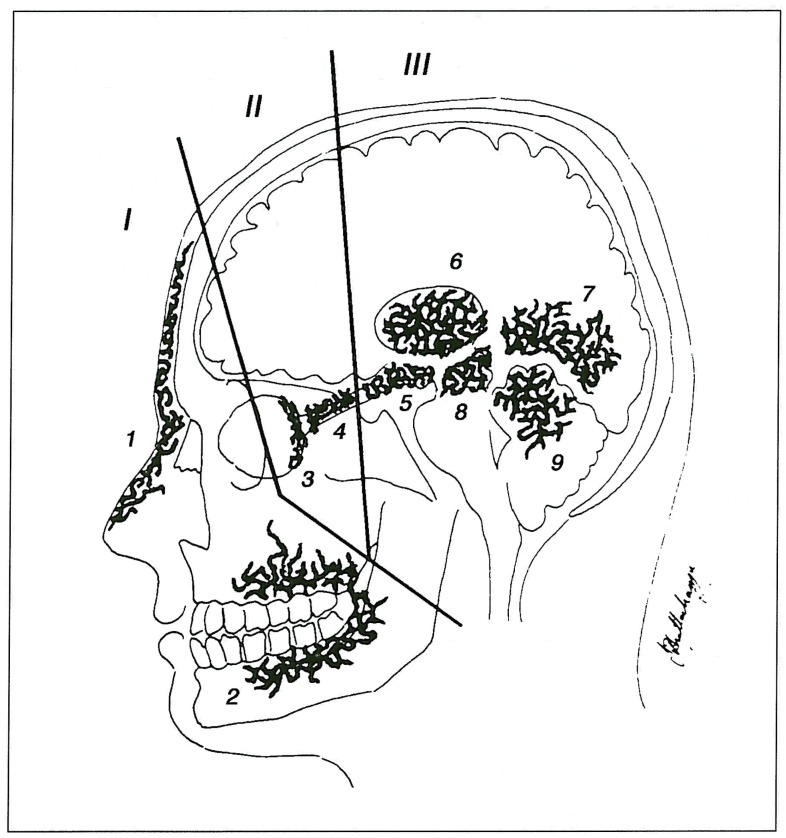
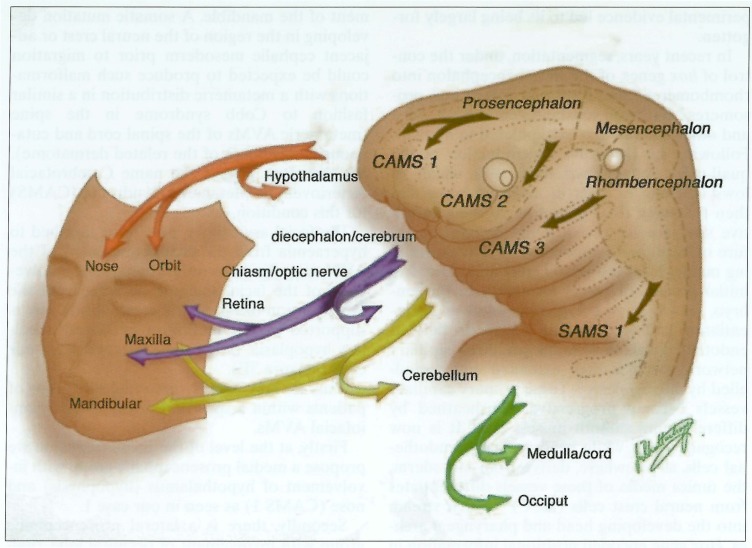
Patient names were obtained from our computer database. Two neuroradiologists reviewed the case records and imaging studies. Demographic details, clinical history and distribution and types of lesions were recorded. There were eight male and seven female patients. Age range at presentation was 4-49 years (mean age 18 years) and 11 of the 15 were 16 years of age or less. All patients were investigated by angiography either at Bicetre or the Institute of Neurological Sciences, Glasgow, or at the referring hospital and most patients also had undergone MRI. The potential areas of involvement are schematically shown in figure 1. As an aid to diagnosis we divide the potential areas of involvement into three zones (brain, orbit and face) and propose the criteria that lesions must be present in at least two of these zones for the diagnosis to be made.
Figure 1.
Schematic drawing showing potential zones of involvement. I: facial; II: orbital; III: cerebral. Locations of lesions within zones: 1) cutaneous; 2) maxillofacial; 3) retina; 4) optic nerve; 5) hypothalamus/chiasm/hypophysis; 6) thalamus; 7) occipital lobe; 8) midbrain; 9) cerebellum.
Results
Patient details with location and types of vascular malformation are summarised in table 1. The age of presentation of these patients (mean age 18 years) is clearly much younger than is seen with sporadic AVMs. The commonest presenting symptom was visual deterioration (reducing acuity or field). This was sometimes detected several years before further neurological symptoms led to more detailed investigations confirming a retinal AVM, with CT or MRI revealing the associated brain AVM. All but one of the 15 patients had orbital involvement while a cutaneous discolouration was only recorded in four cases. Thus the most frequent patterns of involvement were optic nerve 13/15, retina 11/15, thalamus 9/15 and chiasm/hypothalamus 9/15. In our series there was no definite involvement of the midbrain and it is possible that previous reports such as Wyburn-Mason's, suggesting that this was a common site of involvement, confused promi-nent mesencephalic draining veins for nidus, indeed the nidus concept of AVMs was not recognised until 1971, and then initially only in spinal cord lesions6. There were also no cases of cerebellar involvement. Epistaxis was encountered in four patients, in two of whom the source was the associated high-flow maxillofacial AVM (one of these patients - Case 2 - also suffering severe gingival haemorrhage). In the other two no cause was found, and there was no evidence of hereditary haemorrhagic telangiectasia. Four patients suffered intracranial haemorrhage at 4,6,23 and 49 years of age, related to their brain AVM and three of these were treated with embolisation in an attempt to reduce the risk of future haemorrhage. In no case was the brain AVM considered curable. There is no suggestion of an inherited basis of this syndrome with no reports in the literature or in our series of brain or orbital AVMs in relatives, however it was intriguing to find other cerebrofacial vascular lesions in close relatives of two of our patients (see illustrative cases 2 and 3, and discussion).
Table 1.
| No | Age/Sex | Side | Clinical hx | ICH | FH | Cut | FAVM | Retin | OpN | Chi/Hyp | Thal | Stria | OccL | MidB | Cereb | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49/M | Mid | epistax | N | X | X | X | |||||||||
| 2 | 9/F | L | vis,epistax,ging haem | Y | X | X | X | X | X | X | X? | |||||
| 3 | 28/M | Mid(L) | vis,h/a,cosmetic | Y | X | X | X | X | X | X | X | |||||
| 4 | 4/M | L | h/a,seiz,Rt hemi | X | N | X | X | X | X | |||||||
| 5 | 13/M | L | vis,epistax,h/a ? | N | X | X | X | X | ||||||||
| 6 | 8/M | Mid(R)? | vis,Rt hemi | N | X | X | ||||||||||
| 7 | 7/M | R | vis,epistax,h/a,Lt hemi? | N | X | X | X | X | X | Fr,Pr,Tp | ||||||
| 8 | 34/M | L | Rt Hemi,coma | X | N | X | X | X | ||||||||
| 9 | 11/F | L | CHF,HT,?neuro | N | X | X | X | X | X | C an. | ||||||
| 10 | 16/M | Mid(L)? | vis,nasal obstr | N | X | X | X | X? | ||||||||
| 11 | 6/F | L | seiz,ICH/SAH | X | N | X | X | |||||||||
| 12 | 14/F | R | Lt hemi,vis,seiz | N | X | X | X | X | X | |||||||
| 13 | 10/F | Mid(L)? | Rt Hemi | N | X | X | X | X | ||||||||
| 14 | 13/F | L | vis | N | X | X | X | |||||||||
| 15 | 49/M | L | vis,ICH | X | N | X | X | X | ||||||||
|
M - Male; F - Female; Mid = midline; L =left; R = right; h/a = headache; vis = visual symptoms; seiz = seizure; Hemi = hemiparesis; Ging haem = gingival haemorrhage; ICR - intracerebral haemorrhage; SAH = subarachnoid haemorrhage; HT = hypertension; CHF = cardiac failure; epis- tax = epistaxis; FH = family history Sites of lesion: Cut = cutaneous; FAVM = facial AVM; Retin = retinal; OpN = optic nerve; Chi/Hyp = chiasm/hypothalamus; Thai = thalamus; Stria = corpus striatum; OccL = occipital lobe; MidB = Midbrain; cereb = cerebellum; Fr = frontal; Par = parietal; Temp = temporal lobes; C an = ce vical aneurysm; L asym = limb asymmetry | ||||||||||||||||
Illustrative Case Reports
Case 1
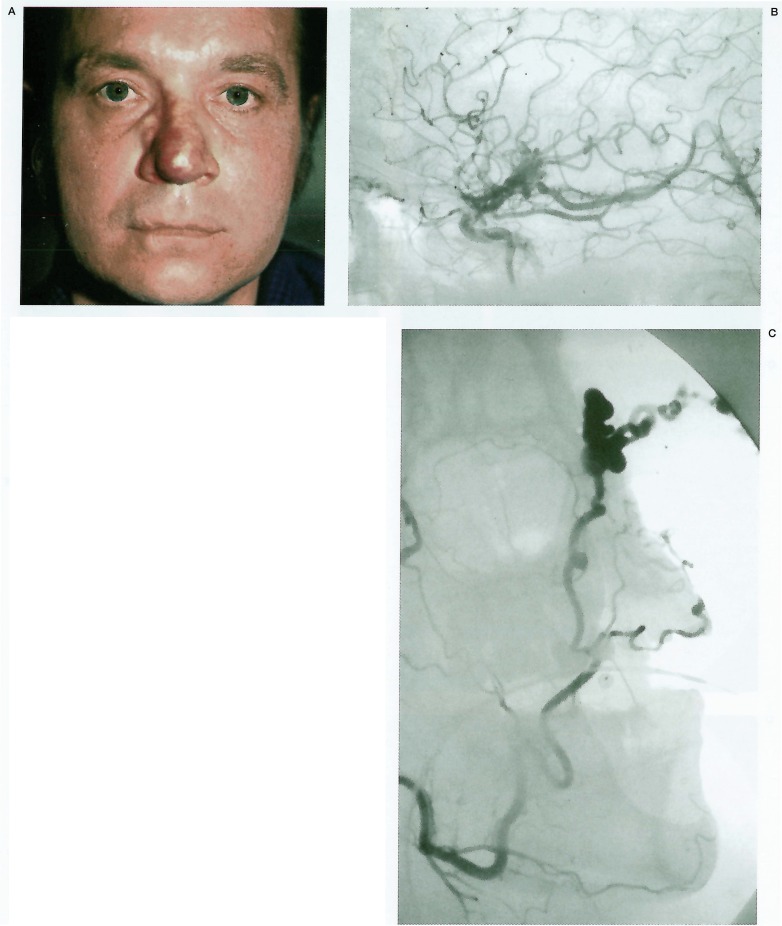
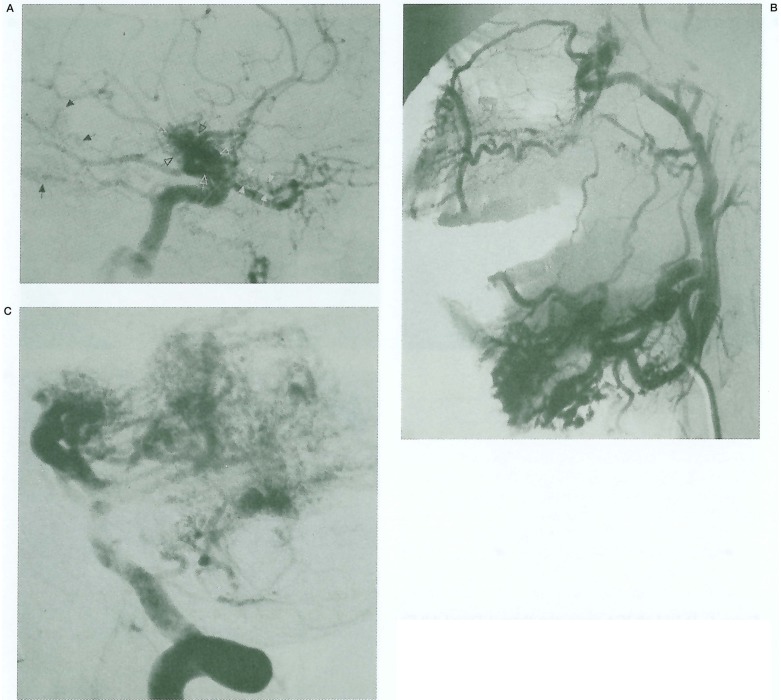
This 49-year-old man was admitted to his referring hospital with severe epistaxis. He had been noted at birth to have an “angioma” of his nose, which had enlarged gradually in recent years. There was no family history of related conditions. Examination revealed a vascular lesion at the tip of his nose (figure 2A), with an arterial bruit suggesting a deeper AVM. He was otherwise well, with normal vision and retinoscopy. He was referred for embolisation of this lesion. External carotid angiography demonstrated a midline nasal and alar AVM (figure 2B) fed by both facial arteries. Internal carotid angiography revealed an additional AVM of the floor of the third ventricle involving the optic chiasm and hypothalamus (figure 2C). Embolisation of the facial artery branches supplying the lesion resulted in successful devascularisation. The hypothalamic AVM was not considered amenable to treatment.
Figure 2.
A) Clinical photograph demonstrates midline angioma of the nose. B) Right ICA angiogram demonstrates hypothalamic AVM. No orbital involvement is seen. C) Facial artery angiogram demonstrates the high-flow nasal AVM.
Case 2
A nine-year-old girl presented originally to her base hospital with the onset of right sided hemiparesis. Investigations revealed an intraventricular haemorrhage arising from an AVM of the left basal ganglia. The hemiparesis gradually improved, no treatment being considered possible. She was reviewed annually and a recent decline in visual acuity was detected in the left eye together with an inferior quadrantanopia of the right visual field.
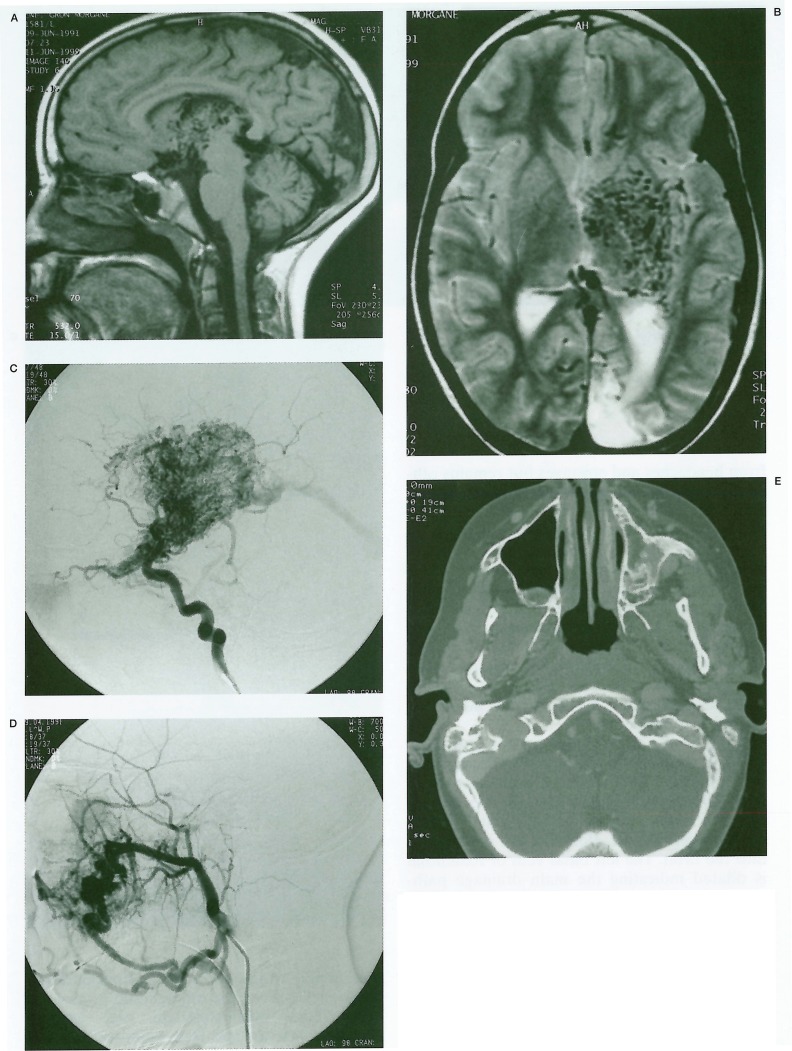
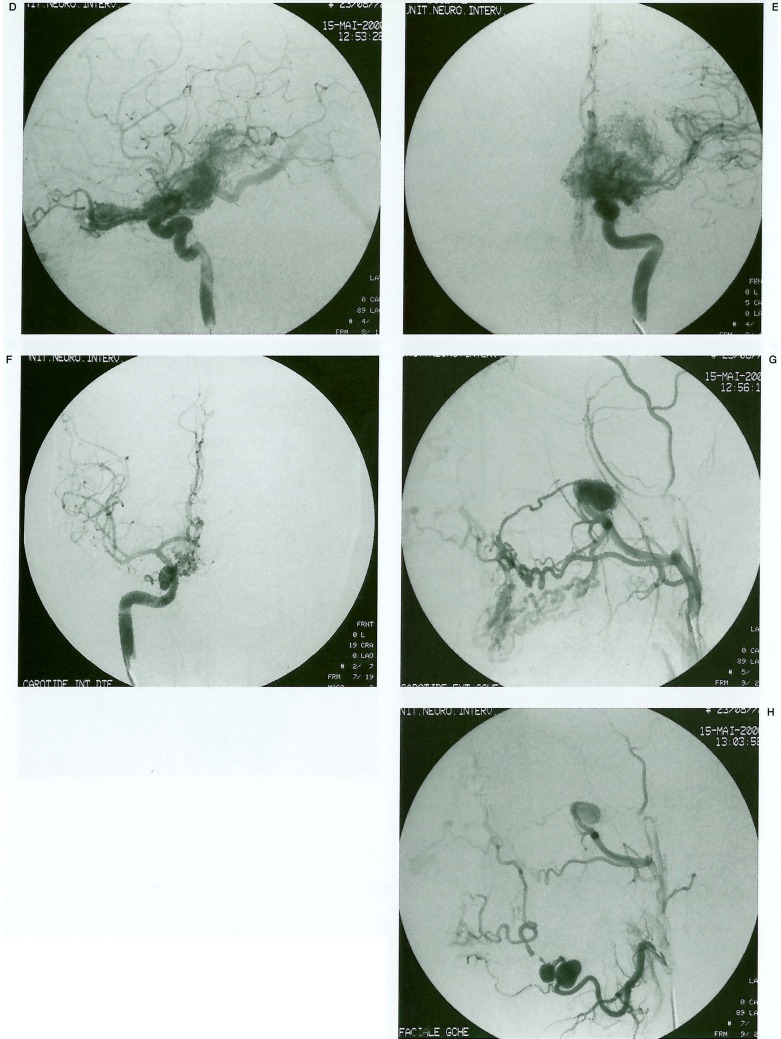
Further investigations were performed at this time, following which she was referred to Bicetre. MRI of the brain (figures 3A, 3B) demonstrated a large unilateral, deeply located AVM of the left hypothalamus, thalamus, internal capsule and lentiform nucleus. The nidus could be seen to extend anteriorly from the lateral geniculate body to the optic chiasm and thence into the left optic nerve. Cerebral angiography demonstrated a large diffuse nidus and extensive surrounding angiogenetic network with arterial supply principally from the left lenticulo-striate, anterior and posterior choroidal and thalamogeniculate arteries (figure 3C). Further supply derived from perforating branches of the middle and posterior cerebral arteries. As seen on MRI, the nidus extended anteriorly along the left ophthalmic artery following the line of the optic nerve and an unusually prominent choroidal blush was observed. Venous drainage was mainly deep, into the internal cerebral veins and vein of Galen. Examination of the external carotid arteries revealed a further arteriovenous malformation of the left maxillary antrum and alveolar ridge, at the level of the 1st and 2nd upper molar teeth, supplied by the left internal maxillary and facial arteries (figure 3D).
Figure 3.
A, B) MRI Brain. A) Sagittal T1 shows large AVM nidus in thalamus and hypothalamus with sparing of the midbrain. B) Axial T2 shows unilateral nidus centred on the deep grey-matter structures. Note also cerebromalacia at left occipital pole (arrow). C) Left ICA angiogram (lat) shows full extent of nidus. Note extension along optic nerve (arrow) and choroidal blush (arrowhead). D) Left external carotid angiogram (lat) shows maxillofacial component of vascular lesion. E) CT of the head on bone windows. Note the hypoplasia of the left maxillofacial skeleton.
These were considered classical features of Bonnet-Dechaume-Blanc syndrome. On clinical review the maxillofacial malformation was noted to be associated with a slight facial asymmetry with hypoplasia of the ipsilateral left maxilla (figure 3E), and on further questioning she was found to have had several previous episodes of gingival haemorrhage. She had no midline facial involvement. There was no family history of similar lesions although a maternal aunt had a capillary haemangioma of the face.
The maxillofacial lesion was considered to present the most immediate danger, with the possibility of life-threatening haemorrhage at the loss of her molar milk teeth. Arrangements were therefore made for embolisation of this part of the lesion-complex.
Case 3
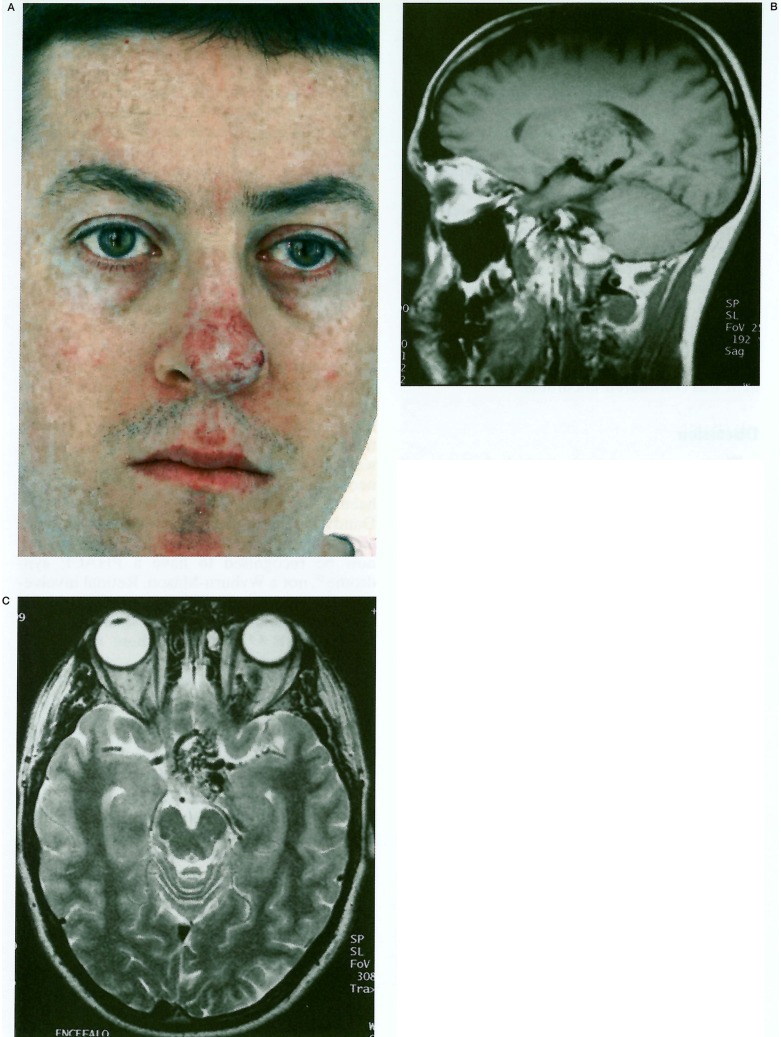
This 28-year-old man was originally found to have a retinal abnormality at the age of seven years. Fluoroscein angiography confirmed the presence of a retinal arteriovenous malformation. More recently CT and MRI studies reportedly showed an arteriovenous malformation involving the left optic nerve, chiasm and thalamus. A diagnosis was made of a retinocephalic vascular malformation syndrome: Wyburn-Mason or Bonnet-Dechaume-Blanc. He is mentally normal, suffers occasionally from headaches and epistaxes but remains otherwise well with no seizures. His sister incidentally also suffers from intermittent epistaxes but is otherwise well and these findings were not felt to support a diagnosis of hereditary haemorrhagic telangiectasia. His mother had a developmental venous anomaly of the occipital lobe diagnosed on CT in the past, but no other abnormality. In 1990 he noticed a small red spot on the tip of his nose, which was diagnosed as an “angioma”. This gradually enlarged causing him cosmetic distress (figure 4A). At this stage he represented to Bicêtre for advice on further management.
Figure 4.
A) Clinical photograph of vascular lesion of the nose. B, C) MRI brain: B) Left parasagittal T1 shows hypothalamic involvement with several flow voids also visible in the thalamus. C) Axial T2 showing midline hypothalamic location of the nidus. Note extension into the left orbit around optic nerve. D, E) Left ICA angiogram (lateral, D and AP, E) shows the elongated nidus. Note the midline location and the partly separate thalamic component. F, G) Left ECA angiogram shows maxillofacial component of the lesion. Note maxillary and nasal AVMs, and aneurysms of the maxillary artery in (F) and maxillary and facial arteries in (G).
MRI of the brain shows a small cerebral AVM in the midline (figures 4B, 4C), its nidus involving the hypothalamus and anterior thalamus, more pronounced on the left side. Anteriorly, the nidus extends into the optic chiasm centrally and thence, along the left optic nerve into the orbit. The left basal vein of Rosenthal is dilated indicating the main drainage pathway. Sagittal images also show flow-voids within the soft tissues of the nose compatible with the vascular lesion here. Cerebral angiography delineated the elongated AVM nidus in the midline (figures 4D, 4E), with a left-sided predominance, spreading from the hypothalamochiasmatic region anterolateral^ into the left orbit, and posterolateral^ into the left thala-mus. Supply to the lesion arises from perforating branches from the supraclinoid portions of both ICAs, lenticulostriate and anterior choroidal arteries and from small branches of the proximal left ophthalmic artery. Perforating branches from the basilar artery contribute modestly to the supply. Principle venous drainage is via a single dilated diencephalic vein into the left basal vein. Injections of the external carotid arteries show two AVMs: one involving the palate and the other the nose, fed by branches of the maxillary and facial arteries (figure 4F). The appearances are typical of Bonnet-Dechaume-Blanc syndrome. In addition a large aneurysm is present at the terminal internal maxillary artery and two further aneurysms cluster together in the facial artery in the submandibular region. Embolisation of the nasal lesion prior to plastic surgery is planned. The other principle threat is the risk of severe epistaxis related to the maxillary aneurysm and embolisation of this will also be discussed with the patient. The brain component was considered uncurable.
Discussion
These cases present typical features of Wyburn-Mason or Bonnet-Dechaume-Blanc syndrome, associating a high-flow arteriovenous malformation of the face with retinal and brain AVMs. In its most extreme form AVMs can extend forwards, continuously, from the occipital lobes and thalamus via the hypothalamus, optic chiasm and optic nerve to the retina4, with midbrain and cerebellar involvement occasionally described 7,8,9 though not seen in our series. Maxillo-facial and cutaneous vascular malformations complete this full expression of what can now be recognised a form of metameric vascular dysplasia. The syndrome is usually classified with the neurocutaneous syndromes or phakomatoses, such as neurofibomatosis, Divry-van Bogaert syndrome, Sneddon syndrome or tuberous sclerosis. This classification however tells us more of our need to classify, than of the nature of the condition itself, the various phakomatoses being of very different morphology and aetiology.
Unlike in neurofibromatosis or tuberous sclerosis, an inherited basis of the condition has never been described although our cases 2 and 3 and a review of the literature do reveal a variety of cutaneous and intracranial vascular abnormalities and anomalies in mostly first degree relatives3,10. These at least raise the possibility that such a basis could exist, although the absence of family history of similar AVMs make a lesion arising “downstream” of a germline disorder probably more likely. The phenotypic expression of the disease is quite variable, our Case 2 for example revealing a far more extensive cerebral AVM component than Case 1. Previous reports have described a range of abnormalities from this phenotypic spectrum, with most stressing the unilateral cerebral involvement. Theron et A17 suggested the alternative name of Unilateral Retinocephalic Vascular Malformation; a unilateral manifestation although most common is not however essential. Indeed Wyburn-Mason described and illustrated with autopsy photographs his Case 84, in which there was a midline lesion of the hypothalamus region with extension to both sides, more marked on the right. The autopsy sections are very close to the MRI appearance of our Case 3, also showing midline localisation (figure 4C) as did our case 1. One case report has shown bilateral orbital involvement11. By contrast, the case report of Gupta et A112, which was reported to show bilateral orbital and cutaneous involvement, illustrates bilateral facial haemangiomas, and the association of the Dandy-Walker malformation which they found is actually far from coincidental: the patient can now be recognised to have a PHACE syndrome 13, not a Wyburn-Mason. Retinal involvement is also not universal, as seen with our case 1, and in three other cases described in two previous reports 14,15. In one of these reports describing two patients 14, there were unilateral cerebral and orbital AVMs with facial involvement (a venous malformation in one case and a maxillofacial AVM in the other). One of these patients had optic atrophy but in neither did fundoscopy identify a retinal AVM.
The exact nature of lesions in earlier descriptions of the facial involvement can be difficult to discern given the wide range of different terms to describe the same lesion. Indeed even in 1937 Bonnet et Al lamented the lack of consistency: La plus grande confusion règne encore dans la dénomination de certaines anomalies vasculaires et ľimprécision de la terminologie contribue à entretenir une certaine obscurité dans la représentation que nous nous en faisons3. The facial involvement varies from complete absence to a faint cutaneous discolouration, from venous malformations to fully-fledged maxillofacial or mandibular AVMs, which may present with life-threatening haemorrhage in addition to severe cosmetic and psychological problems 16. It is notable that only 5/14 of our patients were recorded as having any facial involvement (although this was a retrospective study, and such involvement could have been overlooked or not recorded).
Wyburn-Mason4 in his paper suspected that some previously reported cases of isolated retinal arteriovenous malformation going back to Magnus's1 original may have been part of this syndrome, the neurological component remaining undetected. Both Bonnet et A1 and Wyburn-Mason cautioned that in the presence of a retinal or facial vascular malformation, a possible cerebral AVM should be sought. In his more recent survey of the literature up to 1973, including only cases where the full extent of the abnormality was documented by angiography or pathology, Tʼneron et A17 found that of 25 cases (including his three new ones), four with retinal lesions had no clinical evidence at presentation of a cerebral AVM, and that the ex-tent of facial involvement may not be apparent clinically. This important paper, the most complete survey since the original descriptions, also pointed out that many patients will present to isolated specialists in ophthalmology, otolaryngology, plastic surgery, etc and that the underlying and unifying condition may therefore go recognised. Thus he admonished that a systematic neuroradiological examination be performed when a retinal, or indeed facial vascular malformation is encountered.
Few reports detail the findings of this condition with modern imaging methods. From Northfield's 17 first description of the angiographical appearance, angiography has been the essential examination, as in all cases of cerebral and facial AVM. MRI will however, demonstrate directly involvement of the adjacent brain, and will help guide the aggressiveness of any subsequent therapy. In spite of the absence of high-quality imaging though, the original reports of Bonnet et A1 and Wyburn-Mason remain valid for their elegant and detailed descriptions.
Both of the original reports recognised the singular nature of a lesion complex that appeared to follow the line of the optic pathway from the occipital lobe into the eye, and both considered the lesion complex to be congenital. Wyburn-Mason advanced a surprisingly modern hypothesis regarding the possible embryological origins, although Theron et A1 in 19737 commented that “what factors in development provide for this remarkable specificity are completely obscure”. Actually, the notion of segmentation of the neural tube and adjacent structures of the early embryo, which would provide a rational explanation for involvement of distant but originally related vascular territories, has a long history18, though lack of experimental evidence led to its being largely forgotten.
In recent years, segmentation, under the control of hox genes, of the rhombencephalon into rhombomeres19 and forebrain anlage into prosomeres20 has been substantiated in birds, mice and other animals and extrapolated to humans. Following Le Douarin's 21 introduction of the quail-chick chimera system in 1969, which allows labelling of cells in avian embryos and then following their migration to their definitive sites, studies revealed the metameric nature of brain and craniofacial structures deriving mainly from the neural crest and plate. The initial process of vessel formation in the embryo, known as vasculogenesis involves differentiation and sprouting of mesoderm derived endothelial cells to form the primitive capillary network which is then extended and remodelled by angiogenesis22. These primary capillary vessels become progressively ensheathed by differentiating smooth muscle cells. It is now recognised that while head and neck endothelial cells, as elsewhere, derive from mesoderm, the tunica media of these vessels differentiates from neural crest cells (NCC)23 which stream into the developing head and pharyngeal arches. Hox gene encoded positional information in the crest cells is known to be involved in patterning of the pharyngeal arches and is most likely involved in determining NCC distribution among the arch derived arteries as well24. The work of Couly and Le Douarin has further shown that the neural crest and mesodermal cells originating from a given transverse (metameric) level of the embryo finally occupy the same territory in the head, and that these embryonic tissues are regionalised in various areas devoted to providing blood vessels to specific regions of the face and brain 23.
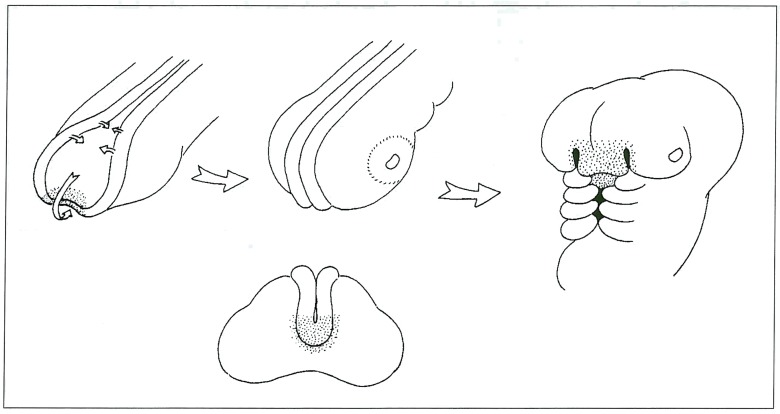
Fate maps of the cells occupying these regions of the neural plate, crest and cephalic mesoderm, in these avian experiments, reveals striking similarities to the distribution of lesions encountered in the human Wyburn-Mason syndrome, for example the region of the anterior lip of the neural plate contains the anlage of the hypothalamus (closely related to the adenohypophysis) and the skin of the future nasal region (figure 5), areas involved in our cases 1 and 3. Similarly, ablation experiments of the anterior rhombocephalic neural crest are associated with absence of development of the mandible. A somatic mutation developing in the region of the neural crest or adjacent cephalic mesoderm prior to migration could be expected to produce such malformations with a metameric distribution in a similar fashion to Cobb syndrome in the spine (metameric AVMs of the spinal cord and cutaneous involvement of the related dermatome). We therefore suggest the name Cerebrofacial Arteriovenous Metameric Syndrome (CAMS) for this condition.
Figure 5.
A) Drawing of the rostrum of an embryo, the arrows indicate progressive closure of the neural folds and curling inferiorly to the anterior lip of the neuropore. Stippled area indicates anlage of the hypothalamus, adenohypophysis and covering nasal tissues. B, C) Oblique and frontal views showing migration of this region. D) Later embryo showing nasal tissues in their final location. Future hypothalamus and adenohypophysis are still just visible prior to closure of the maxillary buds.
The facial asymmetry, previously ascribed to hyperaemia from the facial component of the AVM, may then also reflect metameric involvement of the facial skeleton deriving from the same portion of the neural crest. This theory is supported by our finding of ipsilateral maxillary hypoplasia, rather than hyperplasia, in our case 1 (figure 3E).
Thus we may identify potential subgroups of patients within a spectrum of metameric craniofacial AVMs.
Firstly, at the level of the prosencephalon we propose a medial prosencephalic group with involvement of hypothalamus (hypophysis) and nose (CAMS 1) as seen in our case 1.
Secondly, there is a lateral prosencephalic group with involvement of occipital lobe, thalamus and maxilla (CAMS 2), as seen in our case 2.
The mesencephalic crest does not appear to reach facial expression and lesions here would not be expected to result in a cerebrofacial syndrome.
Next at the level of the rhombencephalon, we predict a third, lateral rhombencephalic group with involvement of cerebellum, pons and mandible (CAMS 3). Although we have not encountered this pattern such a patient was described by Theron et A1 (figure 6).
Figure 6.
Case reported by Tʼneron et Al6 showing (A) brain AVM involving the thalamus and optic nerve, (B) mandible and (C) cerebellum. We classify this lesion complex as CAMS 3. Reproduced with permission.
A more extensive insult would be expected to overlap territories producing mixed phenotypes which could explain our Case 3 (CAMS 1+2). Further, Couly comments that the regionalisation of the cephalic neural crest is not strict and this may allow some fluidity of the final phenotypic expression. This proposed disease spectrum is schematically illustrated in figure 7.
Figure 7.
Schematic drawing of the proposed metameric disease groups (CAMS 1-3), illustrating their main areas of involvement. Note also from the drawing that the upper cervical Cobb syndrome may simply represent the caudal extension of the same disease spectrum: SAMS (Spinal Arteriovenous Metameric Syndrome) 1.
If we continue the schema proposed above, then there is no fundamental difference from the Cobb syndromes of the spinal cord and Cobb would become Spinal Arteriovenous Metameric Syndromes (SAMS) 1, 2, 3... 31 numbered according to the embryonic segment involved.
Interestingly, if we extrapolate to humans, from the avian fate maps, we would predict orbital involvement from all groups 23 and this could be considered the most typical lesion of the syndrome and most common finding in our series, as was believed by the original authors. Of course the disease spectrum could be incomplete either because some cells are spared or because they have not been triggered to reveal the disease 25 and subtle differences between avian and primate crest and cephalic mesoderm migrations may emerge although the basic body plan appears to have been conserved over hundreds of millions of years, and certainly predates the divergence of the mammalian lineage from that of birds and reptiles in the upper paleozoic era. Finally, the insult producing the underlying lesion would have to develop before the migrations occur and thus before the fourth week of development. This supports the possibility that simple sporadic brain AVMs have a similarly early trigger, though they may not be morphologically revealed for several, or even many years25.
References
- 1.Magnus H. Aneurysma arteriovenosum retinale. Arch Path Anat. 1874;60:38. [Google Scholar]
- 2.Yates AG, Paine CG. Case of arteriovenous aneurysm within brain. Brain. 1930;53:38–46. [Google Scholar]
- 3.Bonnet P, Dechaume J, Blanc E. L’anevrisme cirsoide de la retine. (Anevrisme racemeux) Ses relations avec ľanevrysme cirsoide du cerveau. Le Journal Medical de Lyon. 1937;18:165–178. [Google Scholar]
- 4.Wyburn-Mason R. Arteriovenous aneurysm of midbrain and retina, facial naevi and mental changes. Brain. 1943;66:163–203. [Google Scholar]
- 5.Willinsky RA, Lasjaunias P, et al. Multiple cerebral arteriovenous malformations (AVMs): Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology. 1990;32:207–210. doi: 10.1007/BF00589113. [DOI] [PubMed] [Google Scholar]
- 6.Doppman JL. The nidus concept of spinal cord arteriovenous malformations. A surgical recommendation based upon angiographic observations. Brit J Radiol. 1971;44:758–763. doi: 10.1259/0007-1285-44-526-758. [DOI] [PubMed] [Google Scholar]
- 7.Theron J, Newton TH, Hoyt WF. Unilateral retinocephalic vascular malformations. Neuroradiology. 1974;7:185–196. doi: 10.1007/BF00342696. [DOI] [PubMed] [Google Scholar]
- 8.Fischgold H, Bregeat P, et al. Iconographie de ľangiomatose neuroretinienne (syndrome de Bonnet, Dechaume et Blanc) Nouv Presse Med. 1952;60:1790–1792. [PubMed] [Google Scholar]
- 9.Tamaki N, Fumita K, Yamashita H. Multiple arteriovenous malformations involving the scalp, dura, retina, cerebrum and posterior fossa. J Neurosurg. 1971;34:95–98. doi: 10.3171/jns.1971.34.1.0095. [DOI] [PubMed] [Google Scholar]
- 10.Paillas JE, Bonnal J, Righini C. Angiome encephalretino-facial (syndrome de Bonnet, Dechaume et Blanc) Revue Neurol. 1959;101:698–707. [PubMed] [Google Scholar]
- 11.Kim J, Kim OH, et al. Wyburn-Mason syndrome: an unusual presentation of bilateral orbital and unilateral brain arteriovenous malformations. Pediatr Radiol. 1998;28:161. doi: 10.1007/s002470050319. [DOI] [PubMed] [Google Scholar]
- 12.Patel U, Gupta SC. Wyburn-Mason syndrome. A case report and review of the literature. Neuroradiology. 1990;31:544–546. doi: 10.1007/BF00340139. [DOI] [PubMed] [Google Scholar]
- 13.Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, haemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132:307–311. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- 14.Brown D, Hilal SK, Tenner MS. Wyburn-Mason syndrome: report of two cases without retinal involvement. Arch Neurol. 1973;28:67–68. doi: 10.1001/archneur.1973.00490190085013. [DOI] [PubMed] [Google Scholar]
- 15.Gibo H, Watanabe N, et al. Removal of an arteriovenous malformation in the optic chiasm. A case of Bonnet-Dechaume-Blanc syndrome without retinal involvement. Surg Neurol. 1989;31:142–148. doi: 10.1016/0090-3019(89)90329-7. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky M, Hoyt WF, et al. Bonnet-Dechaume-Blanc syndrome with large facial angioma. Arch Ophthalmol. 1987;105:854–855. doi: 10.1001/archopht.1987.01060060144050. [DOI] [PubMed] [Google Scholar]
- 17.Northfield DWC. Angiomatous malformations of the brain. Guys Hosp Rep. 1940-41;90:149–170. [Google Scholar]
- 18.Orr H. Contribution to the embryology of the lizard. J Morphology. 1887;1:311–372. [Google Scholar]
- 19.Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- 20.Puelles L, Rubinstein JLR. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organisation. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- 21.Le Douarin NM, Catala M, Batini C. Embryonic neural chimeras in the study of vertebrate brain and head development. Int Rev Cytol. 1997;175:241–308. doi: 10.1016/s0074-7696(08)62128-2. [DOI] [PubMed] [Google Scholar]
- 22.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 23.Couly G, Coltey P, et al. The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mech Dev. 1995;53:97–112. doi: 10.1016/0925-4773(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 24.Bergwerff M, et al. Neural crest cell contribution to the developing circulatory system. Implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- 25.Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interventional Neuroradiol. 1997;3:275–281. doi: 10.1177/159101999700300401. [DOI] [PubMed] [Google Scholar]