Abstract
Objective
To investigate the antioxidant activity of methanolic extracts of Lantana camara (L. camara) various parts and the determination of their total phenolics content.
Methods
The extract was screened for possible antioxidant activities by free radical scavenging activity(DPPH), xanthine oxidase inhibition activity and Griess-Ilosvay method.
Results
The results showed that all the plant parts possessed antioxidant properties including radical scavenging, xanthine oxidase inhibition and nitrites scavenging activities. The antioxidative activities were correlated with the total phenol. The leaves extract of L. camara was more effective than that of other parts.
Conclusions
This study suggests that L. camara extracts exhibit great potential for antioxidant activity and may be useful for their nutritional and medicinal functions.
Keywords: Lantana camara, Cytotoxicity, Oral acute toxicity, Vero cell, Plant extract
1. Introduction
Oxidative stress is an important risk factor in the pathogenesis of numerous chronic diseases. Free radicals and other reactive oxygen species are recognized as agents involved in the pathogenesis of sicknesses such as asthma, inflammatory arthropathies, diabetes, Parkinson's and Alzheimer's diseases, cancers as well as atherosclerosis. Reactive oxygen species are also said to be responsible for the human aging[1],[2].
An antioxidant can be broadly defined as any substance that delays or inhibits oxidative damage to a target molecule[3]. The main characteristic of an antioxidant is its ability to trap free radicals. Antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases[4]. Herbal plants considered as good antioxidant since ancient times.
Lantana camara L (Verbenaceae) (L. camara) is a significant weed of which there are some 650 varieties in over 60 countries or island groups. Traditional healers have used Lantana species for centuries to treat various diseases. Different parts of L. camara have been used for the treatment of various human ailments such as itches, cuts, ulcers, swellings, bilious fever, catarrh, eczema, tetanus, malaria, tumor, rheumatism and headache[5]–[8]. Hence, the current study was designed to evaluate the antioxidant activity of extracts of different parts of L. camara including root, stem, leaf, flower and fruit by using DPPH scavenging assay, xanthine oxidase inhibition assay, superoxide scavenging assay and determination of total phenolics content.
2. Materials and methods
2.1. Plant samples
Different parts of L. camara were collected from Amanjaya, Kedah, Malaysia, in February 2008. The identity of plant was confirmed by Dr. S. Sudhakaran, associate professor in faculty of applied sciences, AIMST University, Kedah, Malaysia. A voucher with number 11008 was deposited in the herbarium of Biology School, Universiti Sains Malaysia, Penang, Malaysia.
2.2. Extraction procedure
In the laboratory, the different parts of L. camara sample were washed with freshwater and brushed with a soft brush before drying. Cleaned plant material was transferred to oven (ECOCELL) at 50 °C to stay there for 96 h for drying. Then they were powdered by electric blender. Approximately 100 g of different parts of L. camara powder was added to 400 mL methanol and soaked for 4 d. Removal of the plant material from solvents was done by filtration through cheesecloth, and the filtrate was concentrated using a rotary evaporator.
2.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The antioxidant activity of the extracts were determined using the DPPH free radical scavenging assay described by Nithianantham et al.[9] and Zuraini et al.[10] with some modifications. Briefly, the universal bottle was contained 50 µL of L. camara extracts in concentrations from 1 to 5 mg/mL and 5 mL 0.004% (w/v) solution of DPPH was added. The obtained mixture was vortexed, incubated for 30 min in room temperature in a relatively dark place and then was read using spectrophotometer at 517 nm. The blank was 80% (v/v) methanol. Ascorbic acid (Vitamin C) was used for comparison. Measurements were taken in triplicate. DPPH scavenging effect was calculated using the following equation:
 |
where A0 is the absorbance of negative control (0.004% DPPH solution) and A is the absorbance in presence of extract. The results were reported as IC50 values and ascorbic acid equivalents (AAE, mg/g) of L. camara extracts.
2.4. Folin-Ciocalteu method
The total phenolic content of the extracts were determined according to the Folin-Ciocalteu spectrophotometric method[11] with some modifications. To prepare a calibration curve, 0, 1, 2, 3, 5 and 10 mL of the phenol (gallic acid) stock solution (5 mg/mL) was added into 100 mL volumetric flasks, and then diluted to volume with water. From each calibration solution, 0.25 mL was mixed with 1.25 mL of 10-fold diluted Folin-Ciocalteu's phenol (1 mL Folin reagent and 9 mL deioniezed water) reagent and allowed to react for 5 min. Then, 1 mL of 7.5% Na2CO3 solution was added, and the final volume was made up to 5 mL with deionized water. After 1 h of reaction at room temperature, the absorbance at 760 nm was determined by spectrophotometer. The test was done in triplicate. A calibration curve was plotted to determine the level of phenolics in the samples. Same procedure was done for different parts of L. camara extracts in concentrations: 0.1 mg/mL, 0.5 mg/mL and 1.0 mg/mL. The test was done in triplicate. The results were expressed as gallic acid equivalents (GAE, mg/g) of L. camara extract.
2.5. Xanthine oxidase inhibition assay
The xanthine oxidase inhibition assay was done according to the method previously described by Cos et al.[12] and Torey et al[13]. The uric acid production was calculated according to the increasing absorbance at 290 nm. Test solutions were prepared by adding 400 µL xanthine (final concentration 2 mmol/L), 50 µL hydroxylamine (final concentration 0.2 mmol/L), 50 µL EDTA (final concentration 0.1 mmol/L), and 4 µL, 10 µL, 20 µL and 40 µL extract (final concentrations 2, 5, 10 and 20 µg/mL, respectively). The reaction was started by adding 50 µL of xanthine oxidase (final concentration 50 mU/mL). The mixture (total 5 mL) was incubated for 30 min at 25 °C. Prior to the measurement of uric acid production by measuring the UV absorbance at 290 nm, the reaction was stopped by adding 200 µL of HCl 0.58 N.
The uric acid production was calculated from the differential absorbance with a blank solution in which the xanthine oxidase was replaced by buffer solution. A test mixture containing no extract was prepared to measure the total uric acid production.
Xanthine oxidase inhibition activity was expressed as the percentage inhibition of xanthine oxidase in the above assay system, calculated as:
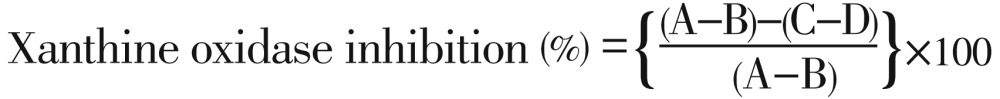 |
Where A is the activity of enzyme without test extract, B the control of A without test extract and enzyme, C and D are the activities of the test extract with and without xanthine oxidase. Allopurinol, a known inhibitor of xanthine oxidase was used as a positive control. The test was done in triplicate. The results were expressed as IC50 values for each part of plant.
2.6. Griess-Ilosvay method
Superoxide levels were measured by the Griess-Ilosvay method[12]. In an acidic solution, nitrite ions are converted to nitrosonium ions; a diazotizing reagent (sulfanilic acid) is added to form a diazonium ion, and this diazonium ion binds with N-(1-naphthyl) ethylenediamine dihydrochloride to produce an azo compound, which is observable as a magenta dye[14].
Same procedure as xanthine oxidase inhibition assay was applied. After stopping the reaction by HCl, the coloring reagent consisting 200 µL sulfanilic acid (final concentration 300 µg/mL), 200 µL of N-(1-naphthyl)- ethylenediamine dihydrochloride (final concentration 5 µg/mL), and 1 mL glacial acetic acid (final concentration 16.7% (v/v) was added. The mixture was allowed to stand for 30 min at room temperature, and the absorbance at 550 nm was measured by spectrophotometer. The test was done in triplicate. Superoxide scavenging effect was calculated as following formula:
 |
Where, Ao is the total absorbance in absence of extract and A is the absorbance of treated extract. The results were recorded as IC50 values of parts of L. camara.
2.7. Statistical analysis
Data were analyzed by SPSS 16.0.0 (SPSS Inc. TEAM EQX). Equations for best fitted line to estimate IC50 values obtained by linear regression statistics based on least squares method. Following one way analysis of variance (ANOVA), treatment means were compared using post hoc comparisons tests. Kruskal-Wallis H nonparametric test was used for examining superoxide scavenging effect data.
3. Results
3.1. DPPH assay
The DPPH radical scavenging activity results are shown in Figure 1 and Table 1 as comparable with known antioxidant Vitamin C. From the analysis of Figure 1, we can conclude that the scavenging effects of leaves, flower, root and stem extracts on DPPH radicals were excellent, especially in the case of L. camara leaves. The RSA values were also remarkably good for flower, root and stem, but L. camara fruits revealed a low value of antioxidant activity. Table 1 shows antioxidant activity with IC50 values of L. camara Vitamin C, leaves, flower, root, stem and fruit measured by DPPH radical-scavenging assays. Overall, L. camara leaves revealed the best antioxidant properties (significantly lower IC50 values=16.02 µg/mL; P<0.05) and the L. camara fruits revealed a very poor antioxidant activity (significantly lower EC50 values=90.11 µg/mL; P<0.05).
Figure 1. DPPH scavenging effect vs. final concentration of L. camara extracts and Vitamin C.
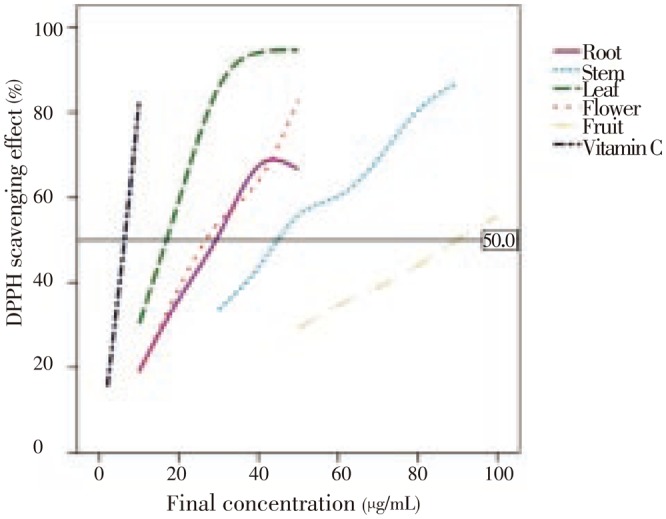
The reference line largely estimates IC50.
Table 1. The IC50 values of DPPH scavenging effect of L. camara extracts (µg/mL).
| Part of L. camara & Vitamin C | IC50 ±SD (DPPH) |
| Vitamin C | 6.21±0.04 |
| Leaf | 16.02±0.94 |
| Flower | 28.92±0.19 |
| Root | 31.52±0.74 |
| Stem | 46.96±2.51 |
| Fruit | 90.11±0.57 |
3.2. Folin-Ciocalteu method
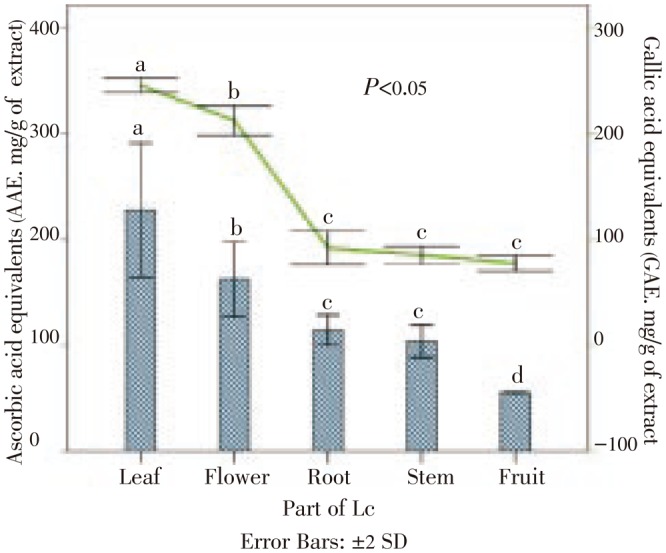
Figure 2 presents total phenol contents obtained for all the L. camara extracts. Among all of the extracts analysed, a significant content of total phenolics (>100 mg/g of extract) were found for all extracts, except for fruit.
Figure 2. Total phenolic content of different parts of L. camara extracts based on ascorbic acid equivalents (AAE, Bars) and gallic acid equivalents (GAE, Line).
3.3. Xanthine oxidase inhibition assay
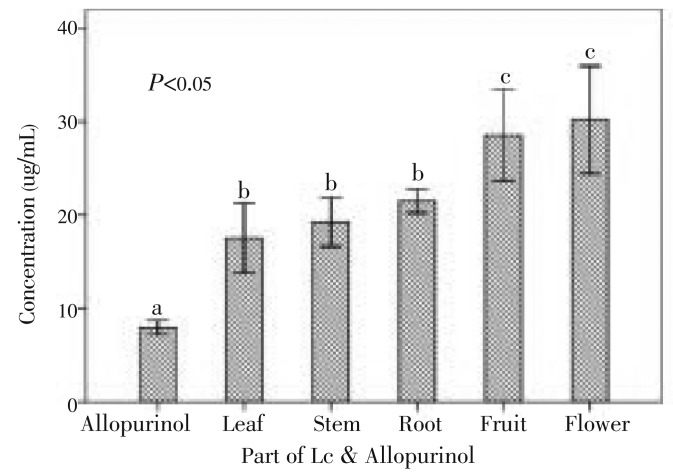
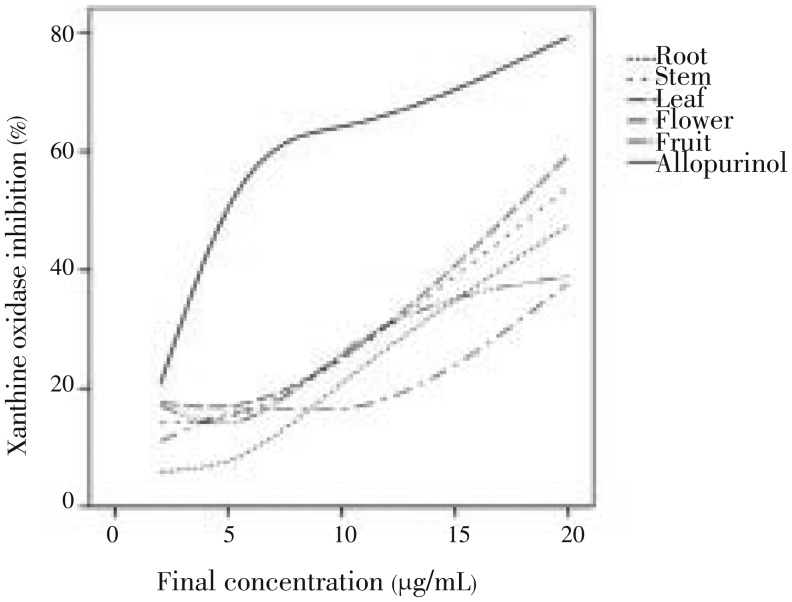
At concentrations of 2.00-20.00 µg/mL, all extracts from leaves, stem and root displayed the inhibition of superoxide formation greater than that of other parts and the xanthine oxidase inhibition effect of all samples was in the order of allopurinol > leaves > stem > root > fruit > flower. In the xanthine-xanthine oxidase system, the IC50 value of all leaves extract was found the highest. Hence, leaves also showed a stronger xanthine inhibition activity than other parts after allopurinol (Figure 3). Figure 4 shows the relationship between xanthine oxidase inhibition and final concentration of the extracts from various parts of L. camara.
Figure 3. IC50 values of different parts of L. camara for xanthine oxidase inhibition. Samples with similar superscripts are significantly similar (P>0.05) checked by Kruskal-Wallis H test.
Figure 4. Relationship between xanthine oxidase inhibition and final concentration of extracts from various parts of L. camara.
The reference line shows the 50% inhibition which is approximately related to the IC50 value of each part.
3.4. Griess-Ilosvay method
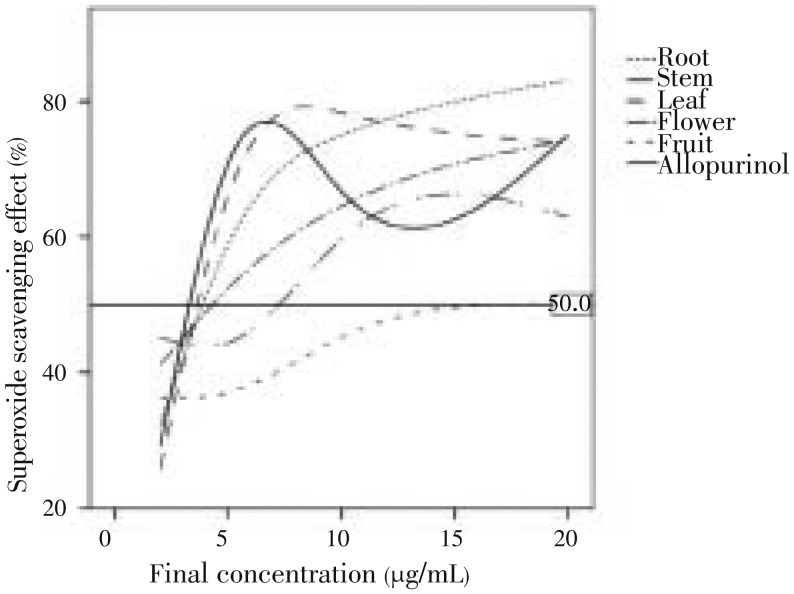
The extract showed potent scavenging activity of nitric oxide with IC50 values in the order of root > leaves > allopurinol > flower > stem > fruit (Figure 5). Figure 6 showed the relationship between nitric oxide scavenging and final concentration of the extracts from various parts of L. camara.
Figure 5. IC50 values of different parts of L. camara and allopurinol for nitric oxide scavenging effect based on Griess-Ilosvay method.
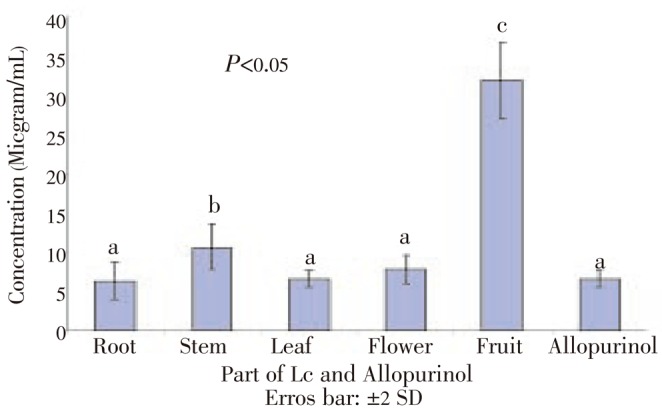
Samples with similar superscripts are significantly similar (P>0.05) checked by Kruskal-Wallis H test.
Figure 6. Relationship between superoxide scavenging effect and final concentration of extracts of L. camara various parts and allopurinol.
The reference line shows the 50% scavenging effect which is roughly related to the IC50 value of each part.
4. Discussion
Antioxidants are tremendously important substances which possess the ability to protect the body from damage caused by free radical induced oxidative stress. The antioxidant potential of L. camara methanol extracts was investigated in the search for new bioactive compounds from natural resources. It became clear that L. camara leaves, flower, root and stem present the highest antioxidant activity compared with reference antioxidant Vitamin C for DPPH scavenging activity. Polyphenols was found in all the samples and in the following order: Leaves > flower > root > stem > fruits. The obtained results for DPPH are in agreement with the phenol contents determined for each sample. Plant polyphenols act as reducing agents and antioxidants by the hydrogen-donating property of their hydroxyl groups[15]. Hence, we could conclude that these polyphenols are responsible for the observed antioxidant activity in this study.
Xanthine oxidase is a flavoprotein, which catalyses the oxidation of hypoxanthine to xanthine and generated superoxide and uric acid[16]. It has exhibited that xanthine oxidase inhibitors may be useful for the treatment of hepatic disease and gout, which is caused by the generation of uric acid and superoxide anion radical[17]. Leave extract exhibited good xanthine oxidase inhibition activity. Interestingly leaves extract also possessed a good DPPH free radical scavenging activity in this study. Hence the leaf extract could be the best candidate to isolates compound with xanthine oxidase inhibition activity. Concerning xanthine oxidase inhibition profile in Figure 4, allopurinol had a sharp rise in xanthine oxidase inhibition at lower concentrations and a slowly increase at higher ones, in contradiction to L. camara extracts with a gradual increase of inhibition at lower concentrations and then rocketing in more ones. The probability of this observation is that the mechanism of allopurinol to repress the enzyme is unlike to one of plant extracts. As allopurinol is a suicide competitive inhibitor of xanthine oxidase[18], the above precondition may imply that L. camara extracts are not competitive inhibitors for this enzyme. Consequently, L. camara extracts may bind to different active sites of xanthine oxidase.
Nitrite ions react with Griess reagent, which forms a purple azo dye. In presence of test components, likely to be scavengers, the amount of nitrites will decrease. The degree of decrease in the formation of purple azo dye will reflect the extent of scavenging[19]. Nitric oxide is produced by several different types of cells, including endothelial cells and macrophages. The early release of nitric oxide through the activity of constitutive nitric-oxide synthase is important in maintaining the dilation of blood vessels the much higher concentrations of nitric oxide produced by inducible nitricoxide synthase in macrophages can result in oxidative damage. Nitric oxide reacts with free radicals, thereby producing the highly damaging peroxynitrite. Nitric oxide injury takes place for the most part through the peroxynitrite route because peroxynitrite can directly oxidize LDLs, resulting in irreversible damage to the cell membrane[19]–[25]. Hence, various extract from L. camara could be used to overcome various health problem causes by nitric oxide injury. Concerning nitric oxide scavenging profile in Figure 6, allopurinol, flower and root had a fluctuated profile in contradiction to stem, fruit and leaves. Galbusera et al.[26] studied the reaction of allopurinol with xanthine oxidase and confirmed that allopurinol produces superoxide radicals during its conversion to oxypurinol. It also failed to scavenge O2• in isolated pig heart[27]. These observations demonstrate the possibility that allopurinol is not a suitable positive control for nitric oxide scavenging effect assay.
These findings show that the L. camara extracts possesses antioxidant activity. DPPH assay revealed that leaf extract had the highest antioxidant activity comparable with Vitamin C (leaf IC50=16.02±0.94 µg/mL; Vitamin C=6.21±0.04 µg/mL). Total phenolics content of leaf extract also highest (245.50±3.54 mg gallic acid/g) which attributed to the antioxidant activity of L. camara's leaf extract in this study. The leaf extract also showed a good xanthine oxidase inhibition activity and was double (IC50 value) than that of allopurinol a known antioxidant. The leaf extract is a promising candidate for use as natural products based antioxidant for the health of human being.
Acknowledgments
This work was partly supported by USM Incentive Grant (Grant Number: 2009/167) From Universiti Sains Malaysia.
Comments
Background
The objective of this study was to investigate the antioxidant effects of extracts of L. camara and the determination of their total phenolics content of various parts of this plants.
Research frontiers
The current study was undertaken to evaluate the antioxidant activity of extracts of different parts of L. camara including root, stem, leaf, flower and fruit by using various in vitro methods and also determination of total phenolics content in the extract.
Related reports
Maria Jancy Ran et al., 2012 reported the phytochemicals present in the L. camara leaves and evaluate antioxidant potential of the ethanolic extract. Total phenol content was estimated by Folin Ciocalteu method and the phenolic content was 17.00 mg/100 of gallic acid equivalent (GE). Antioxidant activity was evaluated by DPPH method and the leaves of L. camara showed 78.21 mg/100 of Ascorbic acid Equivalent Antioxidant Capacity (AEAC). The activity of non-polar chemical constituents from GC-MS study was analysed.
Innovations and breakthroughs
This study has showed that that the L. camara extracts exhibited good antioxidant activity which contributed by phenolics compound.
Applications
The finding of this study suggested that the extract from this plant can be used in the development of antioxidant products.
Peer review
This is a good study in which the authors evaluated the antioxidant activity of extracts of root, stem, leaf, flower and fruit of L. camara. The results are interesting and suggested that L. camara extracts exhibited antioxidant activity which can apply in pharmaceutical products.
Footnotes
Foundation Project: This work was partly supported by USM Incentive Grant (Grant Number: 2009/167) From Universiti Sains Malaysia.
Conflict of interest statement: We declare that we don't have interest of conflict.
References
- 1.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16:2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 2.Chiavaroli V, Giannini C, De Marco S, Chiarelli F, Mohn A. Unbalanced oxidant-antioxidant status and its effects in pediatric diseases. Redox Rep. 2011;16:101–107. doi: 10.1179/174329211X13049558293551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamagishi S, Matsui T. Nitric oxide, a Janus-faced therapeutic target for diabetic microangiopathy-Friend or foe? Pharmacol Res. 2011;64:187–194. doi: 10.1016/j.phrs.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Wu YY, Li W, Xu Y, Jin EH, Tu YY. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. J Zhejiang Univ Sci B. 2011;12:744–751. doi: 10.1631/jzus.B1100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Karam M, Shier WTA. Simplified plaque reduction assay for antiviral agents from plants. Demonstration of frequent occurrence of antiviral activity in higher plants. J Nat Prod. 1990;53:340–344. doi: 10.1021/np50068a011. [DOI] [PubMed] [Google Scholar]
- 6.Afolayan AJ, Meyer JJM. The antimicrobial activity of 3, 5, 7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J Ethnopharmacol. 1997;57:177–181. doi: 10.1016/s0378-8741(97)00065-2. [DOI] [PubMed] [Google Scholar]
- 7.Hernández T, Canales M, Avila JG, Duran A, Caballero J, Vivar AR, et al. et al. Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlán de las Salinas, Puebla (México) J Ethnopharmacol. 2003;88:181–188. doi: 10.1016/s0378-8741(03)00213-7. [DOI] [PubMed] [Google Scholar]
- 8.Chellaiah M, Muniappan A, Nagappan R, Savarimuthu I. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J Ethnobiol Ethnomedicine. 2006;2:43. doi: 10.1186/1746-4269-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nithianantham K, Shyamala M, Chen Y, Latha LY, Jothy SL, Sasidharan S. Hepatoprotective Potential of Clitoria ternatea Leaf Extract Against Paracetamol Induced Damage in Mice. Molecules. 2011;16:10134–10145. doi: 10.3390/molecules161210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuraini Z, Rais A, Yoga Latha L, Sasidharan S, Xavier R. Antioxidant activity of Coleus Blumei, Orthosiphon stamineus, Ocimum basilicum and Mentha arvensis from Lamiaceae Family. Int J Nat Eng Sci. 2008;2:93–95. [Google Scholar]
- 11.Sasidharan S, Aravindran S, Latha LY, Vijenthi R, Saravanan D, Amutha S. In vitro antioxidant activity and hepatoprotective effects of Lentinula edodes against paracetamol-induced hepatotoxicity. Molecules. 2010;15:4478–4489. doi: 10.3390/molecules15064478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Poel BV, et al. et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–76. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- 13.Torey A, Sasidharan S, Latha LY, Sudhakaran S, Ramanathan S. Antioxidant activity and total phenolic content of methanol extracts of Ixora coccinea. Pharm Biol. 2010;48:1119–1123. doi: 10.3109/13880200903490505. [DOI] [PubMed] [Google Scholar]
- 14.Ingrid ALP, Karin P, Staffan H, Rolf GGA. Effects of cocoa extract and dark chocolate on angiotensin-converting enzyme and nitric oxide in human endothelial cells and healthy volunteers-A nutrigenomics perspective. J Cardiovasc Pharm. 2011;57:44–50. doi: 10.1097/FJC.0b013e3181fe62e3. [DOI] [PubMed] [Google Scholar]
- 15.Aberoumand A, Deokule SS. Comparison of phenolic compounds of some edible plants of Iran and India. Pakistan J Nut. 2008;7:582–585. [Google Scholar]
- 16.Zarepour M, Kaspari K, Stagge S, Rethmeier R, Ralf R. Mendel and florian bittner. xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant Mol Biol. 2010;72:301–310. doi: 10.1007/s11103-009-9570-2. [DOI] [PubMed] [Google Scholar]
- 17.Sahgal G, Ramanathan S, Sasidharan S, Mordi MN, Ismail S, Mansor SM. In vitro antioxidant and xanthine oxidase inhibitory activities of methanolic Swietenia mahagoni seed extracts. Molecules. 2009;14:4476–4485. doi: 10.3390/molecules14114476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra S, Jena G, Tikoo K, Mukhopadhyay AK. Preferential inhibition of xanthine oxidase by 2-amino-6-hydroxy-8-mercaptopurine and 2-amino-6-purine thiol. BMC Biochem. 2007;8:8. doi: 10.1186/1471-2091-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayabaskaran M, Venkateswaramurthy N, Babu G, Perumal P, Jayakar B. In vitro antioxidant evaluation of Pseudarthria viscida Linn. Int J Curr Pharm Res. 2010;2:21–23. [Google Scholar]
- 20.Ramalakshmi S, Edaydulla N, Ramesh P, Muthuchelian K. Investigation on cytotoxic, antioxidant, antimicrobial and volatile profile of Wrightia tinctoria (Roxb.) R. Br. flower used in Indian medicine. Asian Pac J Trop Dis. 2012;2(Suppl 1):S68–S75. [Google Scholar]
- 21.Guruvaiah P, Arunachalam A, Velan LPT. Evaluation of phytochemical constituents and antioxidant activities of successive solvent extracts of leaves of Indigofera caerulea Roxb using various in vitro antioxidant assay systems. Asian Pac J Trop Dis. 2012;2(Suppl 1):S118–S123. [Google Scholar]
- 22.Kumar S, Narwal S, Kumar D, Singh G, Narwal S, Arya R. Evaluation of antihyperglycemic and antioxidant activities of Saraca asoca (Roxb.) De Wild leaves in streptozotocin induced diabetic mice. Asian Pac J Trop Dis. 2012;2(3):170–176. [Google Scholar]
- 23.Chandel M, Sharma U, Kumar N, Singh B, Kaur S. Antioxidant activity and identification of bioactive compounds from leaves of Anthocephalus cadamba by ultra–performance liquid chromatography/electrospray ionization quadrupole time of flight mass spectrometry. Asian Pac J Trop Med. 2012;5(12):977–985. doi: 10.1016/S1995-7645(12)60186-2. [DOI] [PubMed] [Google Scholar]
- 24.Poongothai K, Ponmurugan P, Syed Zameer Ahmed K, Senthil Kumar B, Sheriff SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(10):778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 25.Karou SD, Tchacondo T, Ouattara L, Anani K, Savadogo A, Agbonon A, et al. et al. Antimicrobial, antiplasmodial, haemolytic and antioxidant activities of crude extracts from three selected Togolese medicinal plants. Asian Pac J Trop Med. 2011;4(10):808–813. doi: 10.1016/S1995-7645(11)60199-5. [DOI] [PubMed] [Google Scholar]
- 26.Galbusera C, Orth P, Fedida D, Spector T. Superoxide radical production by allopurinol and xanthine oxidase. Biochem Pharmaco. 2006;71:1747–1752. doi: 10.1016/j.bcp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun. 1987;148:314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]