Figure 1. Angiotensin II stabilizes hypoxia-inducible factor-1α in renal medullary interstitial cells.
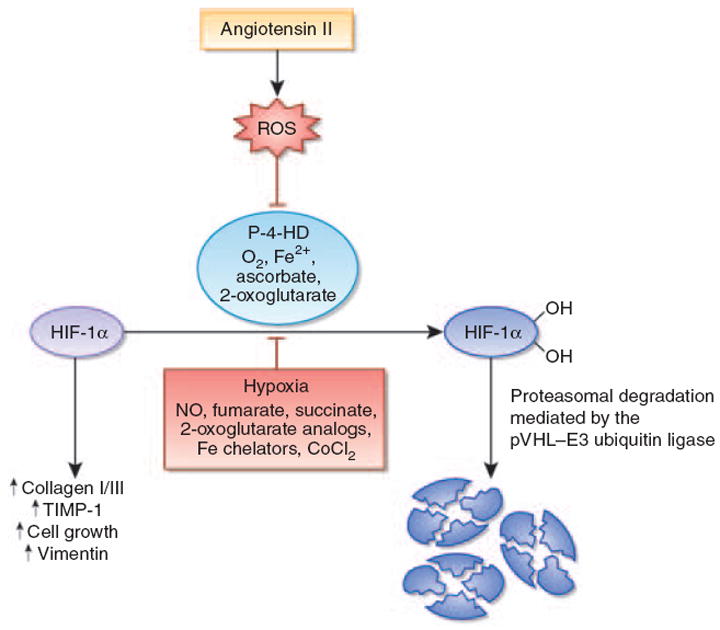
Angiotensin II (Ang II) stimulates the production of reactive oxygen species (ROS). This leads to the inhibition of the hypoxia-inducible factor (HIF)-hydroxylation reaction and subsequent stabilization of HIF-1α. HIF-1α is required for Ang II-induced profibrotic gene expression and proliferation of renal medullary interstitial cells. Under normoxia, HIF-1α is normally hydroxylated by prolyl-4-hydroxylases and targeted for proteasomal degradation by the von Hippel-Lindau (pVHL)–E3 ubiquitin ligase complex. When prolyl-4-hydroxylation is inhibited, HIF-1α is stabilized and translocates to the nucleus, where it heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1α–ARNT heterodimers bind to the HIF consensus-binding site RCGTG, followed by transactivation of target genes. In addition to ROS, nitric oxide, the Krebs cycle metabolites succinate and fumarate, cobalt chloride, and iron chelators such as desferrioxamine inhibit HIF prolyl-4-hydroxylases in the presence of oxygen. CoCl2, cobalt chloride; Fe2+, ferrous iron; NO, nitric oxide; P-4-HD, prolyl-4-hydroxylases; TIMP-1, tissue inhibitor of metalloproteinases-1.
