Figure 10. Working model for the interactions between calreticulin, APP, presenilin and nicastrin.
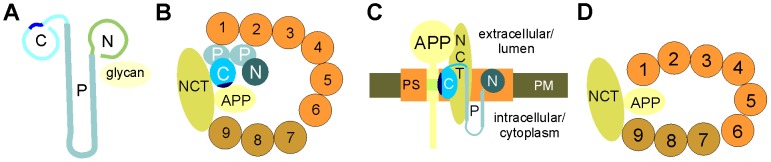
(A) Schematic representation of calreticulin structure (side view): N- and C-domain are globular and the P-domain forms a hairpin-loop structure. The N- and P- domains form the recognition site for glycans. The calreticulin sequence stretch comprising amino acids 330–344 is highlighted (dark blue) (B) Schematic representation of interactions between calreticulin domains, APP, nicastrin (NCT) and presenilin (transmembrane domains are indicated by numbered cycles) in a bird's eye view onto the cell surface. In the water-filled cavity/pore formed by presenilin, APP interacts with the C-domain of calreticulin via amino acids 330–344 (dark blue), while nicastrin interacts with the C- and P-domain of calreticulin. The P-domain interacts with the N-terminal presenilin fragment consisting of the N-terminal transmembrane domains TMD1-TMD6 (circles in orange). The P-domain does not interact with the C-terminal presenilin fragment comprising the C-terminal transmembrane domains TMD7-TMD9 (circles in gold). Nicastrin interacts with TMD9. (C) Schematic representation of the proposed topology of calreticulin domains within the γ-secretase complex in the plasma membrane in a side view of the vertically cut membrane. Nicastrin (NCT) and presenilin (PS; orange box) are depicted in the plasma membrane (PM). The interaction between the amino acids 706–720 of APP within the γ-secretase cleavage sites (light green) and amino acids 334–340 of calreticulin within the C-domain (dark blue) is shown. (D) In the absence of calreticulin, APP can directly interact with nicastrin which functions as substrate receptor for APP as well as with domains TMD1 and TMD9 of presenilin.
