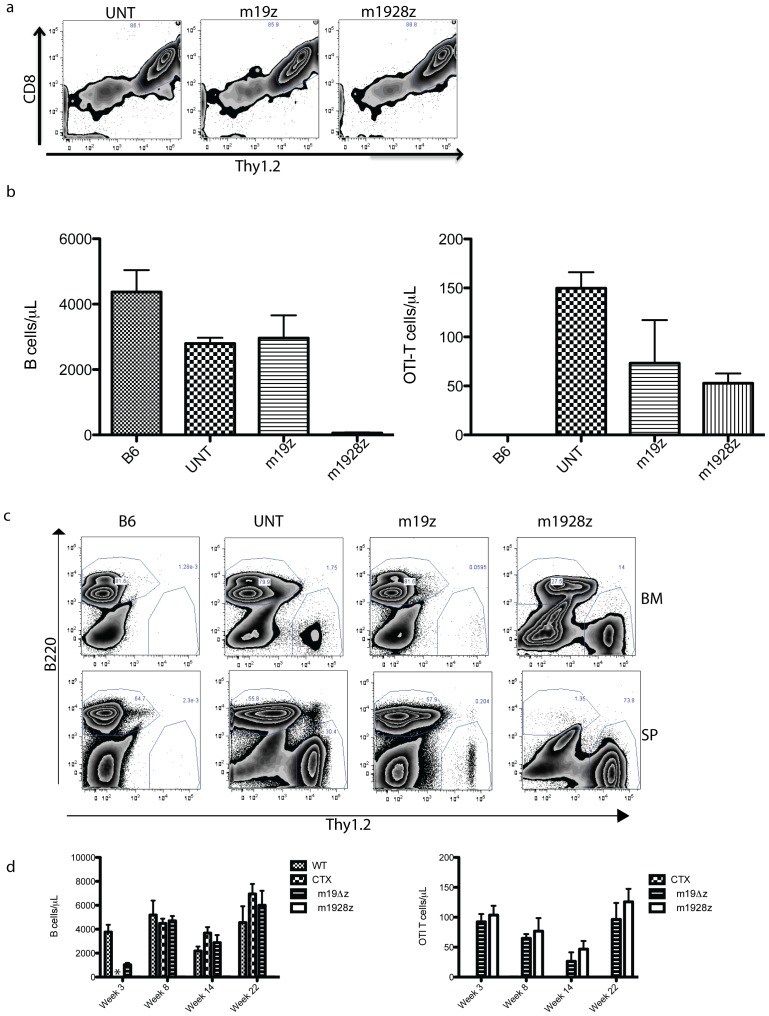
Figure 7. Adoptive transfer of CD8+ CD19 CAR-targeted T cells alone is sufficient for long-term persistence and B cell eradication.
(a) OTI Thy1.2+ T cells were transduced with the m1928z CAR or the m19z CAR, which has a CD3ζ signal transduction domain but lacks the CD28 signal transduction domain. Untransduced T cells (UNT) are mock-transduced cells and are included as a negative control. Donor T cells (5×106) were then adoptively transferred into congenic Thy1.1 mice (n = 19) 1 day after treatment with cyclophosphamide (300 mg/kg). (b) Peripheral blood B and OTI T cell counts were evaluated by flow cytometry 4 weeks after adoptive transfer. One-way ANOVA was done for the treatment groups' (UNT, m19z, m1928z) B cell counts (p = 0.0001) and also OTI T cell counts (p = 0.03). Error bars are the SEM. (c) A few of the mice in each group were sacrificed 7 weeks after adoptive T cell transfer. BM and spleen cells were analyzed by flow cytometry for B and congenic T cell markers. A representative plot is included from each group. Displayed cells were gated on Live and DUMP-negative cells, which were characterized with Mac1, Gr1, NK1.1, and Ter119 antibodies. (d) In another study (n = 17), peripheral blood counts of B and congenic T cells were performed from 3–22 weeks after adoptive transfer. This study included groups of control mice that were untreated wild-type (WT), or treated with cyclophosphamide alone (CTX), or treated with T cells modified with the m19Δz CAR, which lacks any signaling element. Statistical analyses (t tests) of the treatment groups (m19Δz and m1928z) were significant (p<0.05) when done on the B cell counts but not the OTI T cell counts (p = 0.47). Error bars are the SEM. * is Not Done.