Scheme 1.
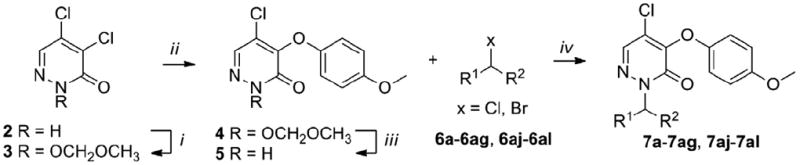
Synthesis of 7a–7ag, 7aj–7al.
Reagents and conditions: (i) 2 (1 equiv.), MOMCl (1.2 equiv.), DMAP (0.1 equiv.), DIPEA (1.4 equiv.) CH2Cl2, 0°C to rt, overnight, 85%; (ii) 3 (1 equiv.), 4-methoxyphenol (1.1 equiv.), NaH (1.1 equiv.), 1,4-dioxane, 15°C to rt, overnight, 65%; (iii) 4 (1 equiv.), BBr3 (1.1 equiv.), CH2Cl2, -78°C to rt, 1h, 93%; (iv) 5 (1 equiv.), 6a-6ag, 6aj-6al (1.2 equiv.), K2CO3 (1.5 equiv.), DMF, rt, overnigt, 40-95%.
