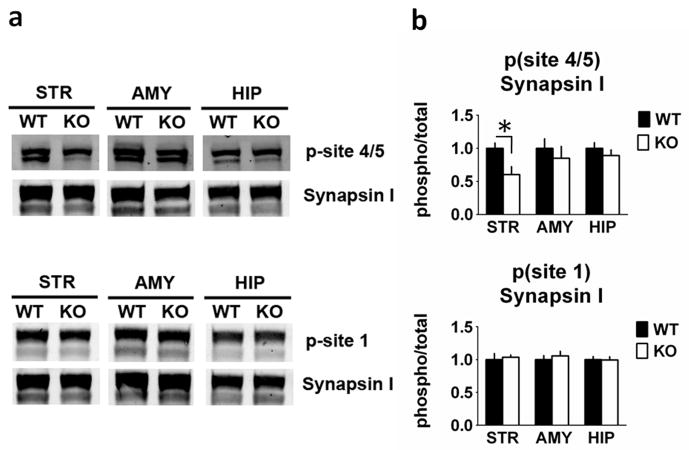
Fig. 7.
Synapsin site 4/5 phosphorylation is reduced in the striatum from RCS knockout (KO) mice. Proteins from striatal (STR), amygdala (AMY) and hippocampal (HIP) extracts were analysed by SDS–PAGE and immunoblotting using synapsin I, phospho-site 1 and phospho-site 4/5 of synapsin I antibodies. Representative immunoblots are shown in (A), and quantitation is shown in (B) and (C). The p-site 4/5 antibody detects Synapsin I as a doublet (isoforms 1a and 1b). The p-site 1 and total Synapsin I antibodies detect 1a/1b as a predominantly single band. Expression levels of phospho-site 1, phospho-site 4/5 of synapsin I were normalized to that of total synapsin I. Error bars indicate SEM, n = 5–7 per group. Synapsin I phosphorylation site 4/5 (Ser62/67) was decreased by 40% in striatal homogenates from RCS KO mice (F1,10 = 7.475, P = 0.021), but was unchanged in amygdala (F1,10 = 0.023, P = 0.88) and hippocampal (F1,10 = 0.005, P = 0.95) homogenates. Synapsin I site 1 phosphorylation was unchanged in all three homogenate samples (amygdala: F1,11 = 0.094, P = 0.77; striatum: F1,11 = 0.499, P = 0.49; hippocampus: F1,10 = 0.392, P = 0.55).