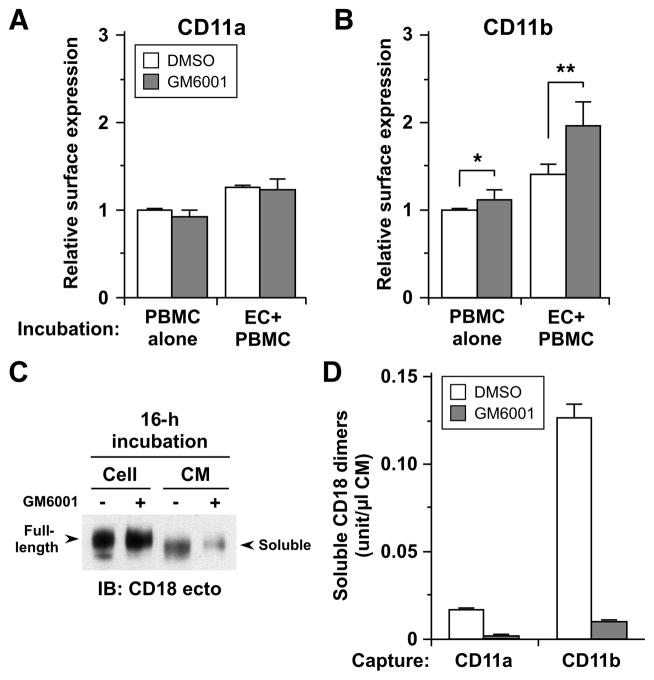
Figure 4. CD18 integrins are shed from human monocytes in a metalloproteinase-dependent manner both constitutively and inducibly upon interaction with endothelial cells.
A and B. Cell-surface expression of CD11a (A), and CD11b (B) on monocytes was determined by FACS analysis after co-incubation with TNF-activated HUVEC monolayers (EC+PBMC) or separate incubation (PBMC alone) for 2 h in the presence of DMSO or GM6001. For analysis of cell surface expression on monocytes, first green-fluorescence endothelial cells were gated out and then CD14+ positive cells were analyzed further. At least 5,000 events were collected. The expression levels are presented as ratios relative to separate incubation with DMSO. Each column represents mean ± SD from 3 different experiments for CD11a, and 5 for CD11b, *p <0.05, **p <0.01 (paired t test). C. Platelet-free monocytes were incubated in Opti-MEM at 106/ml in the presence of DMSO (−) or GM6001 (+) for 16 h at 37°C. Cells were lysed on ice with NP-40 buffer supplemented with proteinase inhibitors and conditioned media (CMs) were concentrated using YM-30 membrane (Millipore) following centrifugation. CMs (40x concentrated, 40 μl) and cell lysates (4 μg) were resolved by 7.5% SDS-PAGE, and evaluated by Western blotting with antibody to the ectodomain of human CD18. Arrows indicate full-length CD18 in the lysates and shed forms (soluble) of CD18 in CM. D. CD18 integrin complexes shed in 16-h CM were quantitated by sandwich ELISA using antibodies for CD11a and CD11b for capture and CD18 for detection. See Table SI for antibodies used.