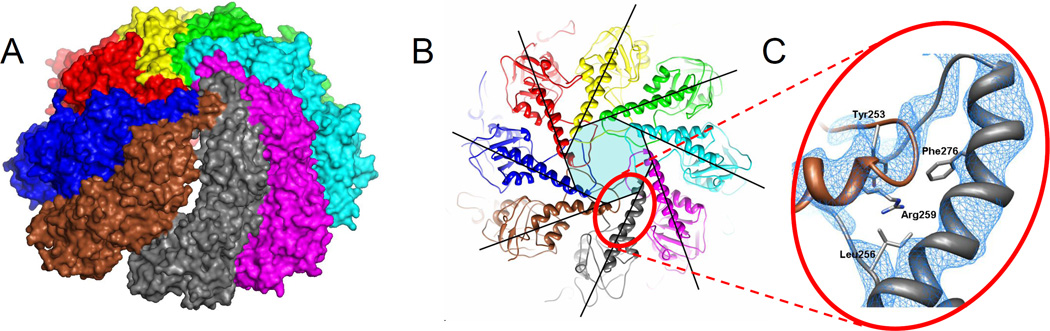
Figure 3. The weak CCT6-Z/CCT8-Q interface in the closed form.
(A) A 'crack' that occurs between these two subunits is not observed elsewhere. Only the top ring is shown. CCT6 is brown, CCT8 is grey.
(B) Top view of the closed form overlaid with a perfect 8-fold iris shows deformation of the Z capping helix. The region around Arg259 of CCT6-Z is marked.
(C) Arg259 is buried in the hydrophobic core of the cap with the guanidinium end group of the side chain exposed on the inner side of the particle. This exerts strain on the Arg259 position that is consistent with the helical deformation. In CCT6 this position is completely conserved across all eukaryotes. The corresponding positions in other subunits are always hydrophobic. The electron density map is averaged over the four rings in the asymmetric unit and contoured at 3σ. See also Fig. S3.