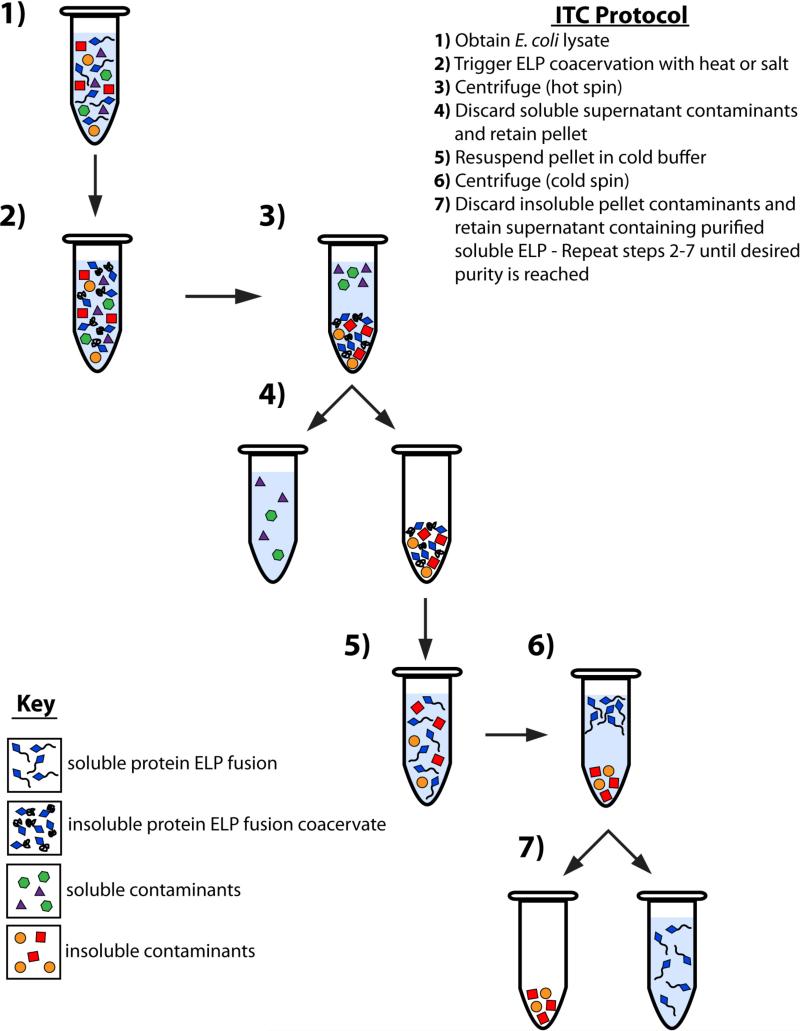
Figure 4. Purification of protein ELP fusions by inverse transition cycling (ITC).
The thermal properties of ELPs provide a means of fusion protein purification that is an excellent alternative to traditional purification methods such as chromatography. E. coli cells are collected from the culture media by centrifugation and lysed by sonication prior to purification by ITC (1). The ELP transition is triggered with the addition of heat or salt (2) and aggregated insoluble ELP is collected by centrifugation (3). The supernatant, containing soluble contaminants, is discarded while the pellet, containing insoluble ELP coacervate, is retained (4) and resolubilized in cold buffer (5). The solution is again centrifuged (6) and the pellet, containing insoluble contaminants, is discarded, while the supernatant, containing the soluble ELP, is retained (7). Steps 2 through 7 constitute one round of ITC, and should be repeated until desired purity is reached.