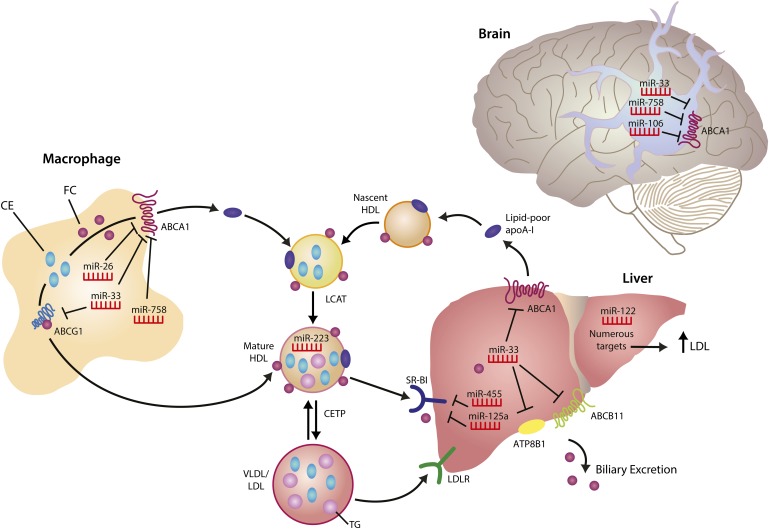
Fig. 1.
Regulation of HDL homeostasis by miRNAs. The liver is the site of HDL biogenesis, where ABCA1-mediated lipidation of lipid-poor apolipoprotein A-I (apoA-I) generates nascent HDL. Free cholesterol (FC) on nascent HDL particles is esterified to cholesteryl esters (CE) by lecithin-cholesterol acyltransferase (LCAT), converting nascent HDL to mature HDL. In turn, ABCG1 mediates cholesterol transfer to mature HDL. CETP mediates CE transfer from HDL to apolipoprotein B-containing lipoproteins (VLDL/LDL) in exchange for triglycerides (TG), promoting plasma cholesterol clearance by uptake of VLDL/LDL lipoproteins through the LDLR pathway. Hepatic SR-BI mediates removal of FC and CE from HDL, through the selective uptake pathway, and excess cholesterol is excreted from the liver into the bile. Both the ABC11 and ATP8B1 transporters promote hepatic clearance, directly via biliary lipid secretion and indirectly via arrangement of adequate canalicular membrane phospholipid asymmetry required for bile salt movement, respectively (net cholesterol movement out of the liver is depicted for simplicity). Numerous miRNAs regulate genes involved in cholesterol efflux and HDL homeostasis in various tissues, including the liver, brain and macrophage. Other microRNAs, such as miR-223, are carried on HDL particles and mediate extracellular signaling by repressing genes in target tissues. Black lines with a right angle at the end represent miRNA target genes that are repressed.