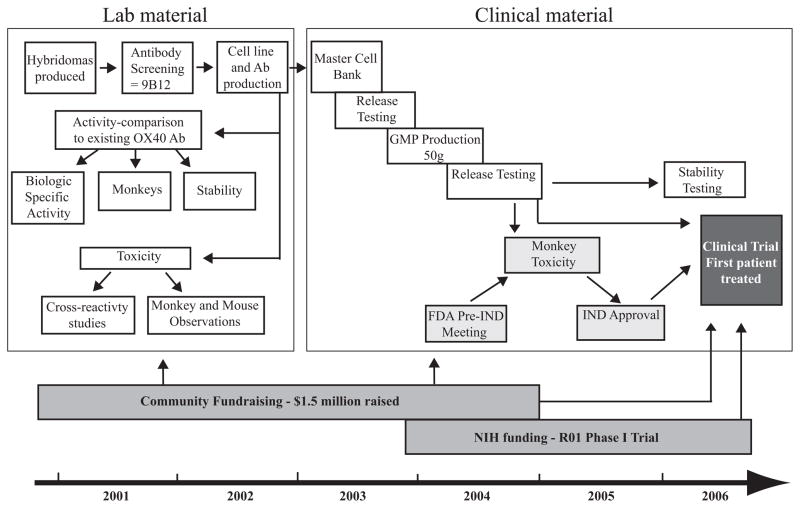
Fig. 2. Timeline for the development of a clinical grade OX40 agonist antibody.
The antibody development steps are depicted in white boxes, regulatory steps in light gray boxes, and funding in dark grey boxes. Characterization of the murine anti-human OX40 agonist monoclonal antibody 9B12 included three separate in vitro potency assays to measure specificity, specific activity, and stability. Initial toxicity and cross-reactivity studies with laboratory scale material indicated that there was little toxicity or off-target binding of the 9B12 Ab. After GMP master cell bank and antibody production had concluded, the FDA mandated that a GLP monkey toxicity study be performed prior to clinical testing. Once the FDA reviewed the monkey toxicity data, the IND was submitted, and the first patient was treated in March 2006. Community fundraising helped support all aspects of the project, and NIH funding supported the phase I clinical trial.