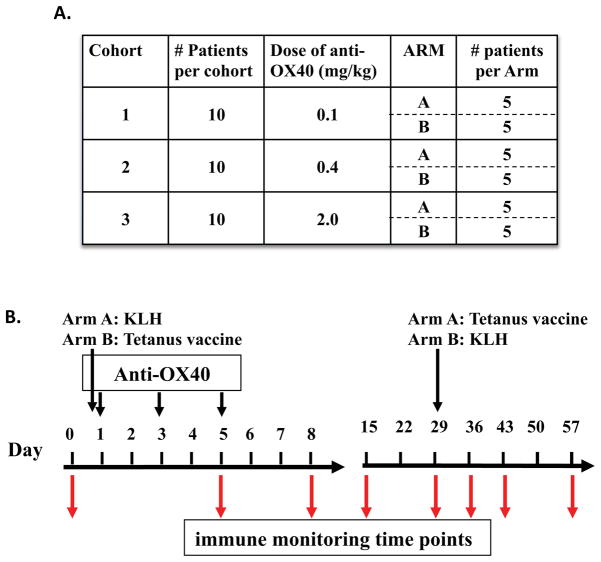
Fig. 3. OX40 agonist clinical trial scheme.
The trial design included 3 dose cohorts with 10 patients treated per dose. As shown in (A), equal numbers of patients within each cohort were randomized into Arm A or B. As shown in (B), patients in Arm A received the reporter antigen KLH on the same day as anti-OX40 administration and tetanus 29 days later, while patients in Arm B received tetanus on the same day as anti-OX40 administration and KLH 29 days later. Anti-OX40 was administered in three separate doses on days 1, 3, and 5, and peripheral blood lymphocytes were isolated at the indicated time points for immune monitoring.