Figure 1.
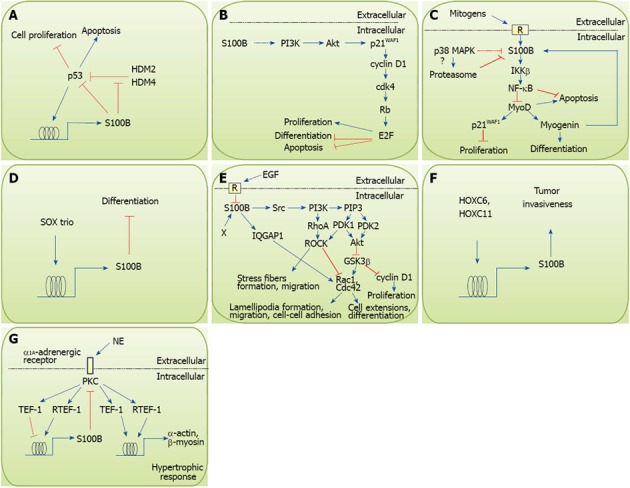
Effects of intracellular S100B on cell proliferation, differentiation, survival and motility. A: Schematics of S100B-p53 interactions in melanoma cells. p53 induces S100B that in turn blunts p53 inhibitory effects on proliferation and stimulatory effects on apoptosis. However, S100B interaction with the ubiquitin E3 ligases, HMD2 and HMD4, may inhibit HMD2/HMD4-dependent reduction of p53 levels; B: Expression of S100B in PC12 neuronal cells results in stimulation of proliferation and inhibition of NGF-induced differentiation and oxidative stress-induced apoptosis via activation of the PI3K/Akt pathway; C: Intracellular S100B protects myoblasts against oxidative stress-induced apoptosis and inhibits differentiation via activation of the IκB kinase β/ nuclear factor κB (NF-κB) pathway. Also, early after the transfer of myoblasts from growth medium to differentiation medium S100B becomes downregulated by the decrease in serum mitogens and activation of the promyogenic p38 MAPK, that likely stimulates S100B proteasomal degradation. However, S100B becomes re-expressed in differentiated myoblasts under the action of myogenin; D: S100B is induced in chondroblasts by the SOX trio and inhibits differentiation; E: S100B, induced in astrocytic progenitors by an unidentified mechanism (X), interacts with and activates a Src/PI3K pathway that stimulates RhoA/ROCK thereby promoting stress fiber formation and cell migration and Akt thereby inhibiting GSK3β resulting in stimulation of proliferation and inhibition of differentiation. Interaction of S100B with IQGAP1 results in activation of Rac1 responsible for lamellipodia formation during migration. The S100B/IQGAP1/Rac1 interaction may also results in an enhancement of cell-cell adhesion as observed in neurospheres (see text). EGF represses S100B expression during early phases of astrocyte differentiation, which appears to be permissive for astrocytic terminal differentiation. Whether such a mechanism also is operating in cerebellar granule cell progenitors remains to be determined; F: S100B, induced in breast cancer cells by HOXC6 and HOXC11, contributes to tumor invasiveness; G: S100B is induced in post-infarction cardiomyocytes by the norepinephrine/α1A-adrenergic receptor/PKC axis and inhibits hypertrophic response via inhibition of PKC
