Abstract
High cardiac vagal control (as measured by respiratory sinus arrhythmia; RSA) is associated with lower depression. Recent theories argue that people’s responsiveness to social resources is a key mechanism in this association. This argument implies two hypotheses: first, high RSA should be related to decreased depressive symptoms only when social resources (social support) are available; second, utilization of available social resources (social engagement) should serve as a mechanism for the positive effects of RSA. To test these hypotheses, we measured RSA in 131 adults. Participants reported their social support, social engagement, and depressive symptoms. Six months later, they again reported their depressive symptoms. Participants with higher RSA reported fewer depressive symptoms six months later, but only under conditions of high social support. The interaction between RSA and social support in predicting depressive symptoms was fully mediated by social engagement. These findings provide crucial support for the idea that cardiac vagal control contributes to decreased depressive symptoms via social processes. Implications for biological sensitivity to context and differential susceptibility theories as well as for the prevention and treatment of depression are discussed.
Keywords: Heart rate variability, Major depressive disorder, Cardiac vagal control, Respiratory sinus arrhythmia, Social support, Social engagement, Person by environment interaction, Psychophysiology
Research has established that vagal influence on the heart (cardiac vagal control) is associated with and causally related to depressive disorders (Beauchaine, 2001; Porges, 2007; Rottenberg, 2007; Taylor, 2010). One particularly important indicator of this vagal influence is respiratory sinus arrhythmia (RSA), which describes the periodic slowing down and speeding up of heart rate with the respiratory cycle (Berntson, Bigger, Eckberg, Grossman, Kaufmann, Malik, et al., 1997). High RSA has been found to be a protective factor against depressive symptoms (Beauchaine, 2001; Rottenberg, 2007). However, the negative associations between RSA and depressive symptoms generally exhibit relatively small effect sizes and are somewhat inconsistent (Rottenberg, 2007). This suggests that the effect of cardiac vagal control on depressive symptoms might be subject to moderating factors. In addition, very little is known about the pathways by which cardiac vagal control may influence depressive symptoms. Thus, identifying moderating factors and mediating mechanisms is of crucial importance for better understanding the association between RSA and depressive symptoms (e.g., Diamond, Hicks, & Otter-Henderson, 2011; Gyurak & Ayduk, 2008; Porges, 2007; Rottenberg, 2007; Rottenberg, Wilhelm, Gross, & Gotlib, 2003; Taylor, 2010).
Respiratory Sinus Arrhythmia and Depressive Symptoms: Conceptual Framework
Several models (e.g., Diamond et al., 2011; Gyurak & Ayduk, 2008; Oveis, Cohen, Gruber, Shiota, Haidt, & Keltner, 2009; Porges, 2007; Smith, Cribbet, Nealy-Moore, Uchino, Williams, MacKenzie, et al. 2011) suggest that social factors are particularly promising to explore as both moderators and mediators of the salutary effects of cardiac vagal control. These theories propose that high RSA is associated with greater responsiveness to environmental stimuli, particularly social ones. More specifically, the polyvagal theory (Porges, 1995, 2007) as well as the neurovisceral integration model (Thayer & Lane, 2000) propose that the same brain circuits that modulate RSA are implicated in behaviors crucial for individuals’ engagement with their social environment, including eye gaze, hearing the human voice, and making appropriate facial and head gestures. Consequently, high RSA may be associated with more flexible responding to the social environment (Butler, Wilhelm, & Gross, 2006; Kashdan & Rottenberg, 2010; Pu, Schmeichel, & Demaree, 2010) and with social engagement (Beauchaine, 2001; Diamond et al., 2011; Kok & Frederickson, 2010; Oveis et al., 2009; Smith et al., 2011).
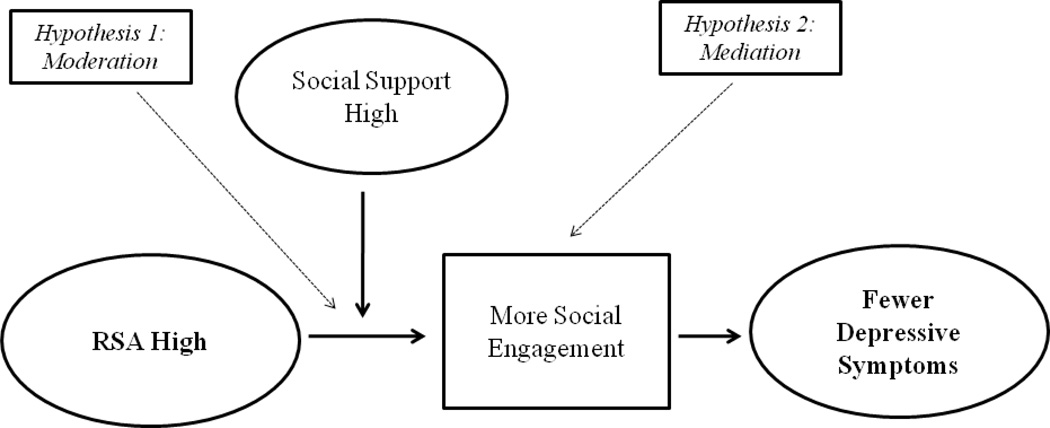
These arguments lead to two hypotheses regarding the link between RSA and depressive symptoms. First, the beneficial effects of high RSA should crucially depend on individuals’ social contexts. This is because greater responsiveness to social cues can only support adaptive social processes in environments with available social resources (Belsky & Pluess, 2009; Porges, 2007). In other words, RSA should predict decreased depressive symptoms but only in environments that have available social resources (i.e., socially supportive environments). A recent experimental study (Wolff, Wadsworth, Wilhelm, & Mauss, 2012) lends initial support to this idea by demonstrating that vagal regulation in children was associated with attenuated stress responding but only under conditions of social support. Thus, theoretical considerations and some previous research indicate that beneficial effects of RSA depend on the social resources available to the individual (see Hypothesis 1, Figure 1).
Figure 1.
Mediated moderation model of the study. High RSA and high social support lead to fewer depressive symptoms (Hypothesis 1: Moderation). Social engagement mediates the relationship between RSA and depressive symptoms under conditions of high social support (Hypothesis 2: Mediation).
The second hypothesis regarding the association between RSA and depressive symptoms is that social engagement should link high RSA to decreased depressive symptoms. This is because high cardiac vagal control is thought to facilitate engagement in shared activities and relationships (Oveis et al., 2009; Porges, 2007; Smith et al., 2011). Thus, more social engagement may be the mechanism by which RSA translates into fewer depressive symptoms (see Hypothesis 2, Figure 1). This idea is supported by research suggesting a) that social engagement depends on RSA (Gyurak & Ayduk, 2008; Kok & Frederickson, 2010; Oveis et al., 2009; Smith et al., 2011) and b) that social engagement is negatively correlated with depressive symptoms (Hawkley & Cacioppo, 2010; McKnight & Kashdan, 2009).
In sum, these considerations suggest a model in which high cardiac vagal control (as measured by RSA) leads to decreased depressive symptoms under conditions of high but not low social support (Hypothesis 1, Figure 1). In addition, the joint effect of high cardiac vagal control and high social support on fewer depressive symptoms should be mediated by social engagement (Hypothesis 2; Figure 1). In statistical terms these hypotheses translate as a mediated moderation model. This model has important implications for understanding the nature and effects of cardiac vagal control as well as, more broadly, for understanding person by environment interactions in predicting depression. However, the hypotheses implied by this model have not yet been examined empirically and as a set.
The Present Study
To test our model, we recruited a community sample of 131 adults aged 21 to 59 years who had recently encountered a stressful life event. Studying individuals recently faced with a wide range of stressful life events allowed us to examine the effects of RSA in a context in which depressive symptoms are pervasive (Boyce & Ellis, 2005; Coifman & Bonanno, 2010). We asked participants to report their social support, social engagement, and depressive symptoms. Afterwards, we measured their RSA in a standardized laboratory setting. Six months later, we again asked participants to report their social engagement and depressive symptoms. By assessing depressive symptoms twice and controlling for initial depressive symptoms when examining effects on later depressive symptoms, we were able to test whether high RSA protects from the development of future depressive symptoms, thus minimizing some of the most severe limitations of cross-sectional designs. Thus, we were able to examine the hypothesized temporal relationships in our model.
Because previous research has established an association between RSA and depressive symptoms (e.g., Rottenberg, 2007) and theorizing suggests a moderating influence of social processes on this relationship (e.g., Porges, 2007), we defined RSA as a predictor for depressive symptoms and examined the moderating role of social support on this association rather than vice versa. To assess whether social engagement explained the hypothesized link between RSA and future depressive symptoms, we tested within-person changes in social engagement (differences between initial assessment and six months later) as a mediator (cf. Lindenberger, von Oertzen, Ghisletta, & Hertzog, 2011). Because factors contributing to social engagement may differ considerably between participants, within-person change in social engagement is better suited to test its potential mediating role than between-person differences in social engagement (Brose, Schmiedek, Lövde‘n, Molenaar, & Lindenberger, 2010). By additionally controlling for between-individual differences in stress levels, we accounted for an important potential confound in the hypothesized relationship between RSA, social support, and prospective depressive symptoms.
Method
Sample
Participants were 156 individuals (51.3% women, 48.7% men) from the Denver Metropolitan Area. Participants were recruited through postings in online bulletins or in public areas such as laundromats and local hospitals. Their average age was 40.1 years (SD = 11.04, Range: 21–59). The ethnic composition was mixed: 84.6% were European-American, 1.3% were Asian-American, 5.1% were African-American, 6.4% were either mixed-race or other, and 2.6% of the sample chose not to identify their ethnicity. Participants reported a wide range of family income levels: 5.1% reported earning less than $10,000 per year, 8.3% between $10,000 and $20,000, 12.2% between $20,000 and $30,000, 14.1% between $30,000 and $40,000, 10.3% between $40,000 and $50,000, 15.4% between $50.000 and $70,000, 16.0% between $70,000 and $100,000, 7.1% above $100.000, and 11.5% did not report their income. This sample allowed us to generalize to individuals from a wide range of ages and socioeconomic backgrounds. Of these participants, 131 (84%) took part in the follow-up assessment six months later. No significant differences revealed between participants who returned to follow-up sessions versus those who did not on the key study variables RSA, social support, social engagement, stress level, and depressive symptoms (ps > .05).
Participants were selected during initial telephone interviews as having experienced at least one stressful life event in the previous eight weeks. A stressful life event was defined to participants during recruitment as an event with a distinct starting point (i.e., a relatively acute instead of a chronic stressor) that had a significant, negative impact on their lives. The most common stressful life events in the sample were: Major change in financial status (84%), changed work situation (81.1%), trouble with employer (44.9%), and serious illness or injury of close family member (33.7%).
All participants gave written informed consent to take part in a study on stress and psychological health. The present research was approved by and performed in accordance with guidelines by the ethics committee of the University of Denver.
Procedure
Participants filled out questionnaires online at home within one week after the initial telephone interview. Questionnaires assessed demographics, perceived negative impact of recent stressful life events, social support, social engagement, and depressive symptoms (see Measures section). Within four weeks of completing the questionnaires, participants were invited to complete individual laboratory sessions to assess RSA. Six months after the laboratory session, participants filled out questionnaires online that again assessed their social engagement and depressive symptoms.
Measures
Respiratory Sinus Arrhythmia
RSA was assessed as heart rate variability within the high frequency band associated with respiration (Berntson et al., 1997; Berntson, Norman, Hawkley, & Cacioppo, 2008). In line with the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) and most studies investigating the relationship between RSA and depression (see Rottenberg, 2007, for a recent review), a frequency band of 0.15 – 0.50 Hz was used for the present study. To compute RSA, heart period (HP) scores computed from Lead-II ECG signals (Biopac Systems, Inc, Santa Barbara, CA) were first converted into time series data with a 4 Hz resolution using cubic spline interpolation. HP time series were first linearly detrended, and the power spectral densities were computed for each baseline period using the Welch algorithm (Welch, 1967), which creates ensemble averages of successive periodograms. Averages were derived from spectra estimated for 60-s data segments, overlapping by half. For each 60-s segment, 256 points were analyzed, which includes 240 sampled points with zero padding. The segments were Hanning windowed and subjected to fast Fourier transform. Estimates of power were adjusted to account for attenuation produced by the Hanning window. Distribution characteristics were normalized by natural logarithm transformation (resulting in a measure often termed lnHF).
We assessed RSA during an emotionally neutral, 2-minute film clip that depicted how sandcastles are built. Similar films have been used successfully to measure physiological activation in an emotionally neutral state (Rottenberg, Ray, & Gross, 2007). RSA was computed, edited and exported using ANSLAB Software, a customized physiological scoring software package (Wilhelm, Grossman, & Roth, 1999; available at the software repository of the Society for Psychophysiological Research, http://www.sprweb.org) that has been used in similar research (Butler et al., 2006; Wolff et al., 2012).
Although some considerations suggest that the association between vagal influence on the heart and RSA is influenced by aspects of respiration (e.g., Grossmann & Taylor, 2007; Wilhelm, Grossman, & Coyle, 2004), we did not adjust for respiration indicators in the present study for two reasons: First, adjusting for respiratory rate is not as useful in between- as in within-subject analyses because people differ considerably in their baseline respiratory rate due to a variety of physiological factors that are not related to cardiac vagal control (e.g., basal metabolic rate, brain stem respiratory pacemaker activity). Thus, in the current analysis, which focuses on differences between individuals in RSA as a predictor for the development of depressive symptoms, respiratory adjustment would not be appropriate. Second, controlling for between-individual differences in respiratory rate would have reduced the comparability of our findings to the bulk of research in the area of RSA and depression, which has not employed adjustments for respiratory rate (Rottenberg, 2007).
Stress Level
We recruited a sample of participants who had recently encountered a stressful life event to increase the overall level and variability of depressive symptoms. To make sure associations between our predictor and outcome variables do not simply reflect the severity of the stressful life events participants encountered, we controlled for between-individual differences in stress levels. To measure stress levels, we used the Life Experiences Survey (Sarason, Johnson, & Siegel, 1978). The Life Experiences Survey consists of 46 items assessing a wide range of life events (e.g., marriage, death of a spouse). For each item, participants indicated if a particular event had occurred within the last 18 months, and its subjective impact with ratings on a 7-point scale, ranging from −3 (= “extremely negative”), to 0 (= “no impact”) and +3 (= “extremely positive”). Perceived impact of stressful life events was assessed rather than more objective aspects (e.g., number of stressful life events) because stress is, in essence, a subjective phenomenon (Lazarus, DeLongis, Folkman, & Gruen, 1985). We measured the impact of events as far back as 18 months because stressful life events can have long lasting, negative impacts on people’s lives. We focused on the negative impact of stressors because negative events are better predictors of psychological health outcomes than positive events (Hopp, Troy, & Mauss, 2011; Lazarus et al., 1985; Sarason et al., 1978). Thus, a total cumulative negative impact score was calculated by summing all impact ratings of negatively rated stressful life events. Summed scores were then reverse coded so that a higher score denotes more cumulative negative impact of stressful life events. We refer to this variable as “stress level.” The present sample included individuals who had experienced a wide range of stress levels (Range: 1–46).
Social Support
We assessed social support by measuring level of perceived social support. Perceived social support reflects subjective judgments that the person’s social environment (e.g., family and friends) will provide assistance if needed, and is typically stable over time (Lakey & Orehek, 2011; Shorey & Lakey, 2011). To obtain an index of perceived social support, participants were asked to answer the 12 items ranging from 1 (= “definitely false”) to 4 (= “definitely true”) of the Interpersonal Support Evaluation List (Cohen, Mermelstein, Kamarck, & Hoberman, 1985). This widely used measure assesses three facets of social support: tangible social support (availability of material aid given by people), appraisal social support (availability of someone to talk to about one's problems), and belonging social support (availability of people one can do things with). Internal consistency (α) of this measure was .90.
Social Engagement
We measured social engagement with items selected from a modified version of the Global Assessment of Functioning (Bodlund, Kullgren, Ekselius, Lindström, & van Knorring, 1994; Davidson, Scott, Schmidt, Tata, Thornton, & Tyri, 2004; Endicott, Spitzer, Fleiss, & Cohen, 1976). Items ranging from 1 (= “not at all”) to 9 (= “extremely”) ask about individuals’ engagement in shared activities and relationships (e.g., “Are you able to keep close friends,” “Do you avoid seeing your friends?” [reverse-scored]). Internal consistencies (αs) of the six items were .74 at the initial assessment and .79 six months later. To test whether within-person changes from the time of the initial assessment to six months later mediated effects of RSA on depressive symptoms six months later, we calculated change in social engagement (social engagement six months later minus initial social engagement, Lindenberger et al., 2011; Skoog & Skoog, 1999). Greater scores indicate increases in social engagement.
Depressive Symptoms
We indexed depressive symptoms using the Beck Depression Inventory (Beck & Steer, 1984), a widely used self-report measure consisting of 21 items, measured at the initial online assessment (α = .92), and six months later (α = .94). Due to ethical concerns, one question regarding suicidal thoughts was omitted from the questionnaire. Means, standard deviations, and intercorrelations of study variables are displayed in Table 1.
Table 1.
Means (M), Standard Deviations (SD), and Intercorrelations of Study Variables
| M | SD | 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Stress Level | 16.49 | 10.15 | - | ||||
| 2 | RSA | 6.69 | 1.39 | −.17* | - | |||
| 3 | Social Support | 3.14 | .68 | −.38** | .13 | - | ||
| 4 | Depressive Symptoms At Initial Levels | 12.51 | 9.50 | .58** | −.20** | −.48** | - | |
| 5 | Depressive Symptoms Six Months Later | 11.59 | 10.00 | .52** | −.22** | −.34** | .68** | - |
| 6 | Change In Social Engagement | .08 | 1.34 | .01 | .04 | −.09 | .04 | −.29** |
Note.
p < .01;
p < .05
Data Analysis
To test whether RSA is associated with fewer prospective depressive symptoms in individuals high, but not low, in social support (Hypothesis 1), we conducted hierarchical regression analyses. We began by centering the predictor (RSA), moderator (social support) and control variables (stress level, initial levels of depressive symptoms), and then multiplied the centered variables for RSA and social support to create a continuous interaction term. In the first step of the regression, we entered our predictor RSA, our moderator social support, as well as our control variable stress level (and initial levels of depressive symptoms, respectively). In the second step, we entered the interaction term of RSA and social support. If the interaction reached statistical significance, we conducted post-hoc simple slope tests among individuals ± 1 standard deviation from the mean of social support.
To examine whether changes in social engagement mediated the joint effect of RSA and social support on prospective depressive symptoms (Hypothesis 2), we followed the procedures for mediated moderation by Muller, Judd and Yzerbyt (2005; see Results section for a detailed description of the conducted analyses).
Results
Hypothesis 1: RSA Predicts Fewer Depressive Symptoms in the Context of High but not Low Social Support
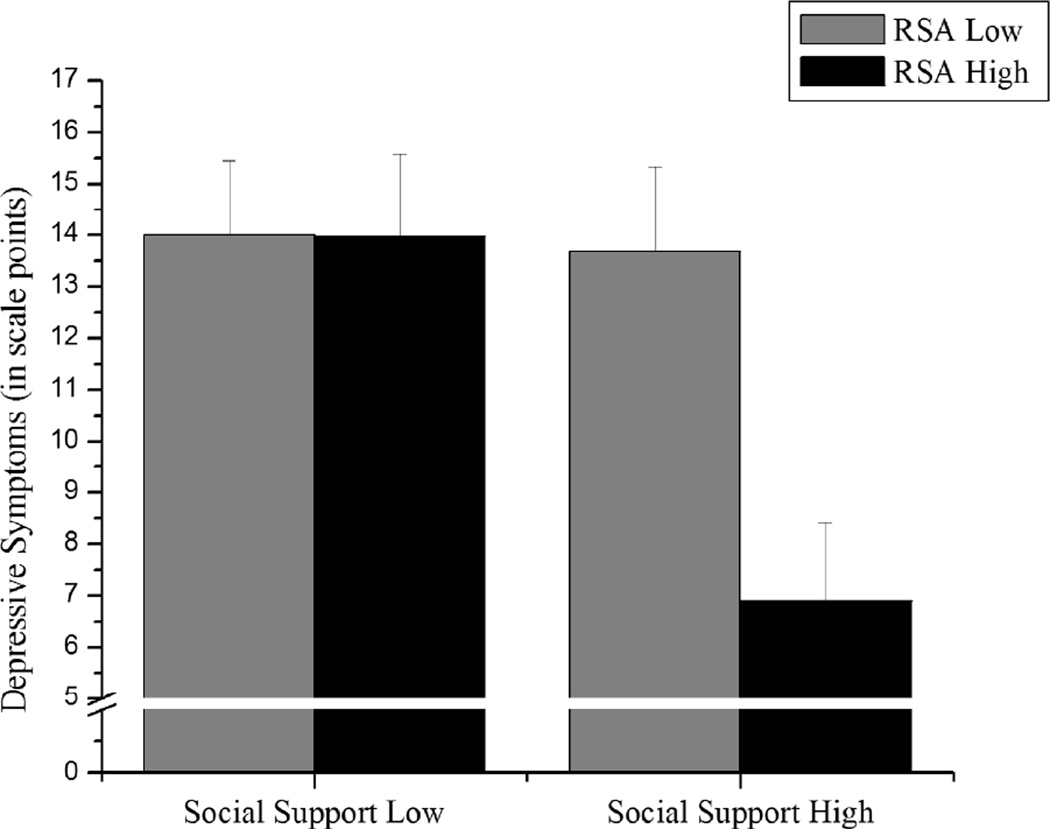
To examine Hypothesis 1, we tested whether the interaction between RSA and social support predicted depressive symptoms six months later. As expected, RSA and social support interacted in predicting depressive symptoms significantly, b = −1.69, p < .039. Also as expected, simple slope analyses revealed that high RSA was related to fewer depressive symptoms six months later when social support was high, b = −3.40, p < .005, but not when social support was low, b = −.02, p < .985 (Figure 2). Furthermore, this prospective effect held when we included initial levels of depressive symptoms into the regression equation, b = −1.69, p < .016 (Table 2).
Figure 2.
High RSA was only associated with fewer depressive symptoms six months after the initial assessment, when social support is high. Regression lines are drawn at ± 1 standard deviation from the means of RSA and social support. Error bars represent standard errors from the mean of ± 1 standard deviation of social support.
Table 2.
Summary of Regression Models Predicting Depressive Symptoms 6 Months Later (with and without Controlling for Initial Levels of Depressive Symptoms) by RSA and Social Support
| Depressive Symptoms Six Months Later | Depressive Symptoms Six Months Later (Controlling For Initial Levels Of Depressive Symptoms) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | |||||
| b | t | b | t | b | t | b | t | |
| Initial Levels Of Depressive Symptoms | - | - | - | - | 5.47 | 6.70** | 5.48 | 6.83** |
| Stress Level | 4.41 | 5.61** | 4.38 | 5.65** | 1.82 | 2.32* | 1.79 | 2.34* |
| RSA | −1.56 | −1.96 | −1.71 | −2.12* | −1.07 | −1.46 | −1.15 | −1.70 |
| Social Support | −1.71 | −2.12* | −1.85 | −2.17* | −.52 | −.72 | −.66 | −.93 |
| RSA×Social Support | - | - | −1.69 | −2.09* | - | - | −1.69 | −2.45* |
| R2 = .32 |
R2 = .34 (ΔR2 = .02*) |
R2 = .50 |
R2 = .52 (ΔR2 = .02*) |
|||||
Note.
p < .01,
p < .05
Hypothesis 2: Social Engagement Mediates the Joint Effect of RSA and Social Support on Depressive Symptoms
To examine Hypothesis 2, we tested whether social engagement mediated the moderated effect of RSA on prospective depressive symptoms following the steps outlined byMuller et al. (2005). First, we tested if the interaction of RSA and social support predicted change in social engagement. As expected, RSA and social support interacted in predicting change in social engagement, b = .34, p < .010 (Table 3)1. Post-hoc analyses showed that high RSA was associated with an increase in social engagement when social support was high, b = .45, p < .021, but not when social support was low, b = −.23, p < .177. Second, we assessed whether change in social engagement predicted depressive symptoms when controlling for the interactions between a) RSA and social support and b) social support and change in social engagement. As expected, change in social engagement predicted depressive symptoms (also when controlling for initial depressive symptoms) while controlling for these two interactions, b = −2.94, p < .001, and b = −3.02, p < .001, respectively. However, the interaction of RSA and social support in predicting prospective depressive symptoms (also when controlling for initial depressive symptoms) was no longer significant while controlling for these interactions (Table 4). Sobel tests indicated that the indirect effect was significant, Sobel’s Z = −2.18, p < .030. This effect was also significant when controlling for initial levels of depressive symptoms, Sobel’s Z = −2.30, p < .022. Thus, change in social engagement fully mediated the moderated relationship between RSA and prospective depressive symptoms.2
Table 3.
Summary of Regression Models Predicting Change in Social Engagement by RSA and Social Support
| Variable | Change In Social Engagement |
|||
|---|---|---|---|---|
| Step 1 | Step 2 | |||
| b | t | b | t | |
| Stress Level | −.02 | −.19 | −.02 | −.12 |
| RSA | .07 | .57 | .11 | .83 |
| Social Support | −.15 | −1.08 | −.12 | −.91 |
| RSA×Social Support | - | - | .34 | 2.62* |
| R2 = .01 |
R2 = .07 (ΔR2 = .05**) |
|||
Note.
p < .01,
p < .05
Table 4.
Summary of Regression Models Predicting Depressive Symptoms 6 Months Later (with and without Controlling for Initial Levels of Depressive Symptoms) by RSA, Social Support, and Change in Social Engagement
| Depressive Symptoms Six Months Later | Depressive Symptoms Six Months Later (Controlling For Initial Levels Of Depressive Symptoms) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 1 | Step 2 | |||||
| b | t | b | t | b | t | b | t | |
| Initial Levels Of Depressive Symptoms | - | - | - | - | 5.63 | 7.41** | 5.66 | 7.43** |
| Stress Level | 4.30 | 5.63** | 4.28 | 5.58** | 1.64 | 2.26* | 1.61 | 2.21* |
| RSA | −1.41 | −1.85 | −1.52 | −1.91 | −.81 | −1.28 | −1.01 | −1.53 |
| Social Support | −2.03 | −2.51* | −2.08 | −2.53* | −.88 | −1.29 | −.87 | −1.26 |
| Change in Social Engagement | −3.15 | −4.36** | −2.94 | −3.92** | −3.26 | −5.45** | −3.02 | −4.88** |
| Social Support×Change in Social Engagement | - | - | .04 | .05 | - | - | .41 | .61 |
| RSA×Social Support | - | - | −.96 | −1.17 | - | - | −.83 | −1.23 |
| R2 = .41 |
R2 = .42 (ΔR2 = .01) |
R2 = .60 |
R2 = .61 (ΔR2 = .01) |
|||||
Note.
p < .01,
p < .05
Discussion
Low cardiac vagal control (as measured by RSA) has long been thought to have pervasive effects on the development of depressive symptoms (Porges, 2007; Rottenberg, 2007). However, some poorly understood inconsistencies in the salutary effects of cardiac vagal control as well as a limited understanding of its mechanisms necessitate more research to develop a full scientific model of cardiac vagal control and depression. The present research provided a theory-driven examination of a moderator and a mediator of the effects of cardiac vagal control on depressive symptoms. Based on theories emphasizing links between cardiac vagal control and engagement with the social context (Porges, 2007; Thayer & Lane, 2000), we expected the effect of high RSA on prospective depressive symptoms to be moderated by social support (social resources available to the individual). Beyond this, we expected the joint effect of high RSA and a socially supportive context on depressive symptoms to be mediated by social engagement (the degree to which individuals utilize social resources available to them).
As predicted, results indicate that high RSA protects people from prospective depressive symptoms depending on the supportiveness of the social environment. Specifically, high RSA translated to fewer depressive symptoms but only in conditions of high social support. Further, social engagement mediated the joint effect of RSA and social support on depressive symptoms six months later. Importantly, all results held when controlling for initial levels of depressive symptoms, suggesting that RSA plays a prospective role in protecting people from the development of depressive symptoms. Thus, our findings support the temporal ordering of our model. Our results appear to be relatively generalizable given that our sample was recruited from the community, displayed a large age range, and had recently faced a wide range of stressful life events.
Implications for Understanding the Relationship between Cardiac Vagal Control and Depressive Symptoms
In concurrently establishing the moderating role of social support and the mediating role of social engagement in the link between high RSA and prospective depressive symptoms, we contribute to a better understanding of the salutary effects of high cardiac vagal control on decreased depressive symptoms. First, the present study demonstrates the importance of considering moderating context variables like social support in the relationship between cardiac vagal control and depressive symptoms. So far, observed effects of RSA on depressive symptoms have been relatively small and inconsistent (Rottenberg, 2007). Results of the present study suggest that high RSA only protects against depressive symptoms if the environment offers higher levels of social resources. Conversely, if social support is low, high RSA may not be effective in countering depressive symptoms (Coifman & Bonanno, 2010; Hawkley & Cacioppo, 2010; Porges, 2007).
Second, in establishing social engagement as a mediating mechanism, we illuminate how high cardiac vagal control translates into decreased depressive symptoms. Thus far, little is known about the mechanisms that link cardiac vagal control to depression (Rottenberg, 2007). In the present investigation we were able to identify social engagement as an intervening process that enables high RSA to protect against the development of depressive symptoms in socially-supportive contexts. Thus, our findings help to better understand the mechanisms by which high cardiac vagal control leads to positive effects.
Wider Theoretical Implications
The present study contributes to development of theory about cardiac vagal control in several important ways. Our findings support predictions derived from the polyvagal theory (Porges, 2007) and the neurovisceral integration model (Thayer & Lane, 2000). By showing that RSA is associated with depressive symptoms only in the context of high social support and by identifying social engagement as the mechanism for this link, the present results provide crucial support for theories hypothesizing that cardiac vagal control has influences on health outcomes via social pathways. In particular, our results are in line with polyvagal theory’s assumption that a “social engagement system” links the neurophysiological processes and neuroanatomical structures involved in social engagement behaviors directly to vagal regulation of the heart. Taken together, the present results are consistent with the core idea from the polyvagal theory (Porges, 2007) and the neurovisceral integration model (Thayer & Lane, 2000) that individuals’ effective engagement with their social context plays a central role in the functions and effects of the vagal system (Beauchaine, 2001; Gyurak & Ayduk, 2008; Smith et al., 2011).
More broadly, the present findings contribute to theories emphasizing that the interplay of person-based (i.e., cardiac vagal control) and environmental factors (i.e., social support) – rather than either factor alone – can be a powerful predictor of symptoms of psychological health (Moffitt, Caspi, & Rutter, 2006). More specifically, biological sensitivity to context (Belsky & Pluess, 2009) and differential susceptibility (Boyce & Ellis, 2005) theories posit that some individuals are more susceptible than others to environmental conditions, and that to fully understand individual differences in health, one must take into consideration theoretically-motivated person by environment interactions. Our findings provide evidence for one important, theoretically-motivated person (cardiac vagal control) by environment (social support) interaction that shapes depression. Moreover, our findings suggest a specific behavioral mechanism (social engagement) for these effects, thereby further extending the scientific understanding of the interaction between cardiac vagal control and social support.
Implications for Interventions
Our findings also have important implications for interventions aimed to prevent the development of depressive symptoms. Although individual differences in RSA show temporal stability (Sloan, Shapiro, Bagiella, Gorman, & Bigger, 1995) and RSA in depressed individuals does not appear to change with current antidepressant medication (Kemp, Quintana, Gray, Felmingham, Brown, & Gatt, 2010), recent research suggests that RSA can be improved through repeated practice (Nolan, Kamath, Floras, Stanley, Pang, Picton, et al., 2005; Sandercock, Bromley, & Brodie, 2005; Tang, Ma, Fan, Feng, Wang, Feng, et al., 2009). Interventions that increase RSA could possibly enhance social behavior, reduce stereotypical behaviors, and improve vocal communication, thereby increasing social engagement (Porges, 2007). As such, one promising avenue in the treatment of depression is vagus nerve stimulation (e.g., Martin & Martin-Sanchez, 2012). However, our results suggest that it might be most useful to administer such interventions in combination with psychosocial interventions that enhance individuals’ social resources, such as cognitive-behavior therapy (CBT). A study that examined the effect of CBT for depression on RSA did not find significant increase of RSA after treatment (Taylor, Conrad, Wilhelm, Strachowski, Khaylis, Neri, et al., 2009). Thus, it might be necessary to include intervention modules aimed to specifically increase the availability of social resources in the environment (e.g., modifying the social network; Hawkley & Cacioppo, 2010; Stokols, 1992). This could protect high-risk individuals against the development of depressive symptoms and enhance existing psychosocial treatments for depression, especially for those with high RSA.
Limitations
We also acknowledge three potential limitations of our study. First, because we did not experimentally manipulate RSA, social support, or social engagement, our findings do not allow for strong causal claims. However, our prospective design supports the temporal ordering of our model which strongly suggests that high RSA in a socially supportive context is a protective factor against the development of depressive symptoms.
Second, the measurement of RSA (as an indicator of cardiac vagal control) employed in the present study may be subject to some confounding factors such as individual differences in respiration. Recently, there has been some debate of how to quantify RSA (see e.g. Lewis, Furman, McCool, & Porges, 2012, for one opinion). Similarly, Grossman and Taylor (2007) mentioned the caveat that RSA may under some circumstances show low sensitivity as an individual-difference variable, particularly when investigating within-subject changes of RSA, e.g., from baseline to stress tasks. However, RSA measured at baseline, like in our study, is commonly used as a between-subject predictor of psychological and physiological health and has shown much utility in this respect (e.g., Carney, Freedland, & Veith, 2005; Giese-Davis, Wilhelm, Conrad, Abercrombie, Sephton, Yutsis, et al., 2006). Importantly, between-subjects variability in RSA has been widely used to predict depression effectively and is a valid measure of a meaningful individual difference (e.g., Rottenberg, 2007; Taylor, 2010, for recent reviews). Because the present study focuses on individual differences in baseline levels of RSA, we believe that our findings add an important contribution to identifying intervening variables in the association between RSA and the development of depression. However, future research might merit from investigating the association between RSA and depression using other ways of quantifying RSA.
Third, because our theoretical model suggested statistical analyses on continuous rather than categorical measures of depression, no structured diagnostic interviews were conducted. Thus, our data do not allow for commenting on incidence rates of major depression and comorbid disorders in our sample. However, the Beck Depression Inventory is a well-validated clinical instrument for assessing the severity of depression (Taylor, 2010). Accordingly, supplemental analyses of our data indicate that 34 % of participants at the initial assessment and 35.1% of participants six months after were in the range of 14 to 48 score points, indicating mild to severe depression (conservative estimates due to the omission of the suicide item in response to concerns of the ethics committee). Thus, we believe that the depressive symptoms assessed in the present sample are clinically relevant.
Concluding Comment
In sum, the present results elucidate how the interplay of RSA, social support, and social engagement shapes future depressive symptoms, and thereby help to clarify the role of the vagal pathway in the development of depression.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (1R21AG031967) awarded to I.B.M. and by a fellowship within the Postdoc-Programme of the German Academic Exchange Service (DAAD) awarded to H.H.
Footnotes
Additional analyses not controlling for stress level revealed that the interaction effects of RSA and social support in predicting prospective depressive symptoms controlling for initial levels of depressive symptoms and in predicting change in social engagement are still significant (p = .016, and p = .009, respectively). However, when not controlling for stress level, the interaction effect of RSA and social support predicting prospective depressive symptoms without controlling for initial depressive symptoms is only marginally significant (p = .053).
Three participants were breathing outside of the respiration band of .15 to.50 Hz as measured by respiratory plethysmography. Analyses excluding these participants did not change the reported results.
References
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. Journal of Clinical Psychology. 1984;40(6):1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(6):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bodlund O, Kullgren G, Ekselius L, Lindström E, von Knorring L. Axis V — global assessment of functioning: evaluation of a self-report version. Acta Psychiatrica Scandinavica. 1994;90(5):342–347. doi: 10.1111/j.1600-0447.1994.tb01604.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. an evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brose A, Schmiedek F, Lövdén M, Molenaar PCM, Lindenberger U. Adult age differences in covariation of motivation and working memory performance: Contrasting between-person and within-person findings. Research in Human Development. 2010;7(1):61–78. [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic Medicine. 2005;67(1):29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: theory, research and applications. Dordrecht: Martinus Nijhoff Publishers; 1985. pp. 73–94. [Google Scholar]
- Coifman KG, Bonanno GA. When distress does not become depression: emotion context sensitivity and adjustment to bereavement. Journal of Abnormal Psychology. 2010;119(3):479–490. doi: 10.1037/a0020113. [DOI] [PubMed] [Google Scholar]
- Davidson K, Scott J, Schmidt U, Tata P, Thornton S, Tyri P. Therapist competence and clinical outcome in the prevention of parasuicide by manual assisted cognitive behaviour therapy trial: the POPMACT study. Psychological Medicine. 2004;34:855–863. doi: 10.1017/s0033291703001855. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Individual differences in vagal regulation moderate associations between daily affect and daily couple interactions. Personality and Social Psychology Bulletin. 2011;37(6):731–744. doi: 10.1177/0146167211400620. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale: a procedure for measuring the overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosomatic Medicine. 2006;68(5):675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Ayduk O. Resting respiratory sinus arrhythmia buffers against rejection sensitivity via emotion control. Emotion. 2008;8(4):458–467. doi: 10.1037/1528-3542.8.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine. 2010;40(2):218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp H, Troy AS, Mauss IB. The unconscious pursuit of emotion regulation: implications for psychological health. Cognition and Emotion. 2011;25(3):532–545. doi: 10.1080/02699931.2010.532606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review. 2010;30(7):865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kok BE, Frederickson BL. Upward spirals of the heart: autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biological Psychology. 2010;85(3):432–436. doi: 10.1016/j.biopsycho.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey B, Orehek E. Relational regulation theory: a new approach to explain the link between perceived social support and mental health. Psychological Review. 2011;118(3):482–495. doi: 10.1037/a0023477. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, DeLongis A, Folkman S, Gruen R. Stress and adaptational outcomes: the problem of confounded measures. American Psychologist. 1985;40(7):770–779. [PubMed] [Google Scholar]
- Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: are commonly used metrics equivalent. Biological Psychology. 2012;89(2):349–364. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what’s change got to do with it? Psychology and Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Martin JLR, Martin-Sanchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. European Psychiatry. 2012;27(3):147–155. doi: 10.1016/j.eurpsy.2011.07.006. [DOI] [PubMed] [Google Scholar]
- McKnight PE, Kashdan TB. The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clinical Psychology Review. 2009;29(3):243–259. doi: 10.1016/j.cpr.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Perspectives on Psychological Science. 2006;1(1):5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Muller D, Judd CM, Yzerbyt VY. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89(6):852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- Nolan RP, Kamath MV, Floras JS, Stanley J, Pang C, Young QR. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. American Heart Journal. 2005;149(6):1137. doi: 10.1016/j.ahj.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9(2):265–270. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Schmeichel BP, Demaree HA. Cardiac vagal control predicts spontaneous regulation of negative expression and subsequent cognitive performance. Biological Psychology. 2010;84(3):531–540. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biological Psychology. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Ray RD, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment. New York: Oxford; 2007. pp. 9–28. [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40(1):1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- Sandercock GRH, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Medical and Science in Sports & Exercise. 2005;37(3):433–439. doi: 10.1249/01.mss.0000155388.39002.9d. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. Journal of Consulting and Clinical Psychology. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Shorey RC, Lakey B. Perceived and capitalization support are substantially similar: implications for social support theory. Personality and Social Psychology Bulletin. 2011;37(8):1068–1079. doi: 10.1177/0146167211406507. [DOI] [PubMed] [Google Scholar]
- Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56(2):121–127. doi: 10.1001/archpsyc.56.2.121. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Gorman JM, Bigger JT., Jr Temporal stability of heart period variability during a resting baseline and in response to psychological challenge. Psychophysiology. 1995;32(2):191–196. doi: 10.1111/j.1469-8986.1995.tb03311.x. [DOI] [PubMed] [Google Scholar]
- Smith TW, Cribbet MR, Nealy-Moore JB, Uchino BN, Williams PG, Thayer JF. Matters of the variable heart: respiratory sinus arrhythmia response to marital interaction and associations with marital quality. Journal of Personality and Social Psychology. 2011;100(1):103–119. doi: 10.1037/a0021136. [DOI] [PubMed] [Google Scholar]
- Stokols D. Establishing and maintaining healthy environments. American Psychologist. 1992;47(1):6–22. doi: 10.1037//0003-066x.47.1.6. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Fan Y, Feng H, Wang J, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(22):8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor CB. Depression, heart rate related variables and cardiovascular disease. International Journal of Psychophysiology. 2010;78(1):80–88. doi: 10.1016/j.ijpsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Conrad A, Wilhelm FH, Strachowski D, Khaylis A, Spiegel D. Does improving mood in depressed patients alter factors that may affect cardiovascular disease risk? Journal of Psychiatric Research. 2009;43(16):1246–1252. doi: 10.1016/j.jpsychires.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Welch PD. The use of the fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Transactions on Audio and Electroacoustics. 1967;15:70–73. [Google Scholar]
- Wilhelm FH, Grossman P, Coyle MA. Improving estimation of cardiac vagal tone during spontaneous breathing using a paced breathing calibration. Biomedical Science Instrumentals. 2004;40:317–324. [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Roth WT. Analysis of cardiovascular regulation. Biomedical Sciences Instrumention. 1999;35:135–140. [PubMed] [Google Scholar]
- Wolff BC, Wadsworth ME, Wilhelm FH, Mauss IB. Children’s vagal regulatory capacity predicts attenuated sympathetic stress reactivity in a socially supportive context: evidence for a protective effect of the vagal system. Development and Psychopathology. 2012;24(2):677–689. doi: 10.1017/S0954579412000247. [DOI] [PubMed] [Google Scholar]