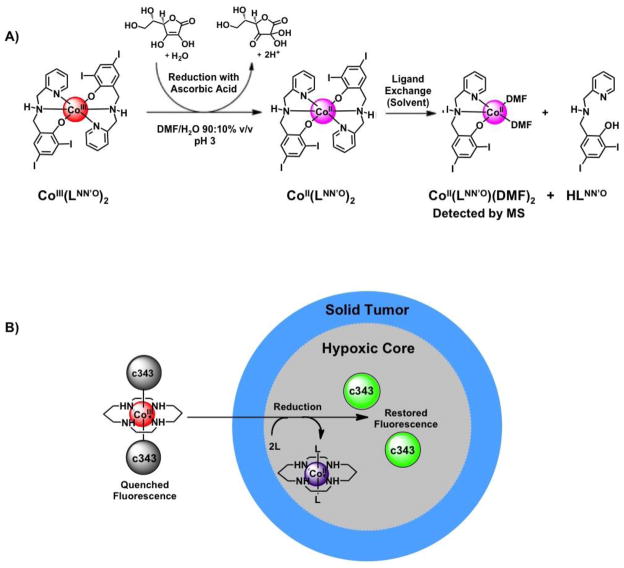
Figure 4.
Cobalt complexes that undergo bioreductive activation. A) Co(III)(LNN′O)2 undergoes reduction to the more labile Co(II) counterpart to yield an active proteasome inhibitor [40,41*]. In the presence of the reducing agent ascorbic acid, one of the LNN′O ligands of Co(III)(LNN′O)2 is lost and replaced by a solvent (DMF) molecule, as identified by mass spectrometry. The activated complex is thought to bind to the active site of the proteasome to elicit inhibition. Scheme adapted from ref [31] with permission. B) The fluorescence of coumarin-343 (c343) is quenched upon coordination to Co(III)/cyclam. When the complex encounters the reducing environment of a hypoxic spheroid core, the complex undergoes reduction, releasing the fluorophores and restoring fluorescence. The process is monitored by fluorescence confocal microscopy [51*].