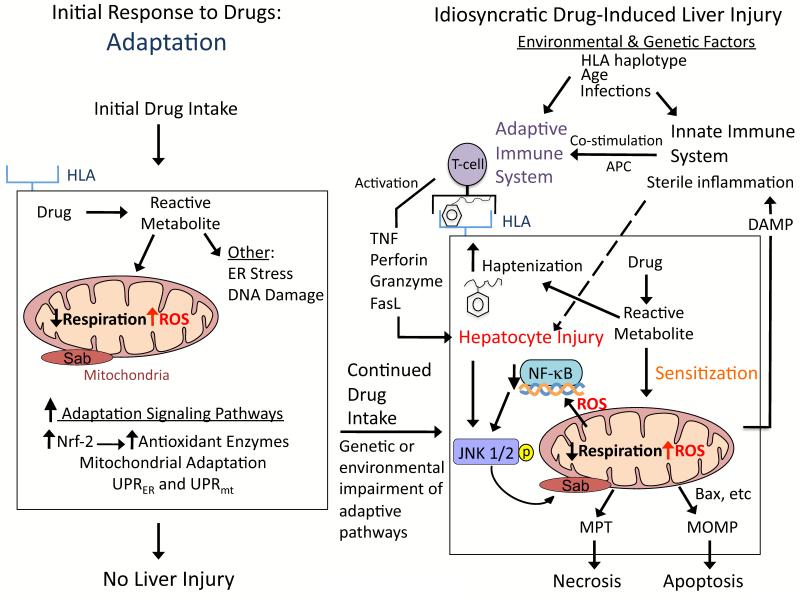
Figure 4. Hypothesis for idiosyncratic DILI.
Signaling pathways that modulate hepatocellular injury during idiosyncratic DILI probably occur in multiple waves following drug intake. The first signaling wave occurs as hepatocytes are exposed to the drug for the first time. The stress induced by the drug/reactive metabolite, in many cases due to disruption of mitochondria, causes activation of signal transduction pathways associated with adaptation to help hepatocytes cope with continuous drug intake. For most patients the activation of adaptation pathways such as Nrf-2 helps hepatocytes adjust to the drug, and no liver injury occurs. However in a minority of patients, due to genetic and environmental factors, the adaptation pathways may be overwhelmed and/or the stress imposed by continuous drug intake may cause signaling changes that sensitize hepatocytes to injury by extrinsic factors, such as the innate and/or adaptive immune system. According to the hapten model, hapten-peptides are processed and presented on HLA binding grooves of antigen-presenting cells (APC), which interact with T-cell receptors on CD4 T-cells. CD4 T-cells are activated and subsequently activate cytotoxic CD8 T-cells (CTL) that express surface FasL and release TNFα, perforin, granzyme and other cytotoxic factors. The activated CTL then target and kill the hepatocytes expressing the hapten peptide on HLA. Stressed hepatocytes may become injured because the drug/reactive metabolite inhibits pro-survival signaling pathways, such as the transcription factor, NF-κB, to sensitize hepatocytes to the immune system. If the stress and injury reach a critical threshold, then cell death signaling pathways including JNK become dominant and hepatocytes enter a failsafe mode of self-destruction. Activated JNK targets mitochondria to promote MPT and MOMP that induce apoptosis or necrosis, which manifests as DILI.