Case Report
A 12 year old girl was referred to QECH Paediatric and Child Health Department with a two week history of dry cough, fever and chills. Three days before admission she became pale and short of breath. At the onset of the symptoms, she was given antimalarial tablets (lumifanterine/artimether) at her local health centre but symptoms worsened. Three days prior to admission when pallor was noted, she was referred to a district hospital where diagnoses of severe anaemia and pneumonia were made. She was transfused 440mls of whole blood and started on intravenous penicillin. She was referred to QECH after 2 days due to recurrent severe anaemia and worsening pneumonia.
She was previously well with no history of shortness of breath on exercise, orthopnoea or chronic cough and no relevant past medical history. There was no family history of congenital heart disease.
On arrival, she looked unwell, in severe respiratory distress, with an oxygen saturation of 64% in air. She was wasted with a body weight of 27kg. Her axillary temperature was 38.6°C, pulse rate was 128b/min and respiratory rate 88 cycles/min. She was pale, had bilateral coarse crepitations in the chest, a gallop rhythm, a 4cm tender hepatomegaly, 3cm splenomegaly and normal looking skin. She had no subungual petechiae or Janeway lesions.
On admission differential diagnoses included severe anaemia (unknown cause), heart failure and severe pneumonia.
Initial investigations were: haemoglobin 5.3g/dl, WBC 23x103/mm3 (lymphocytes 2.70×103/mm3, monocytes 0.80×103/mm3, granulocytes 12.5 ×103/mm3) and platelets 23 × 103/mm3. Microscopy of a thick blood film for malaria parasites, HIV antibody test (Determine™ Trinity Biotech plc, Bray Ireland) and Mantoux,(Tuberculin, Purified Protein Derivative RT 23 SSI, Denmark) were all negative. She was started on IV penicillin, gentamicin and frusemide. She received a blood transfusion and was put on oxygen therapy.
On day 2, her respiratory distress and oxygen requirements increased. She remained in heart failure. Blood cultures were taken. Chest x-ray showed bilateral fluffy infiltrates with an enlarged cardiac shadow (Figure 1).
Figure 1.
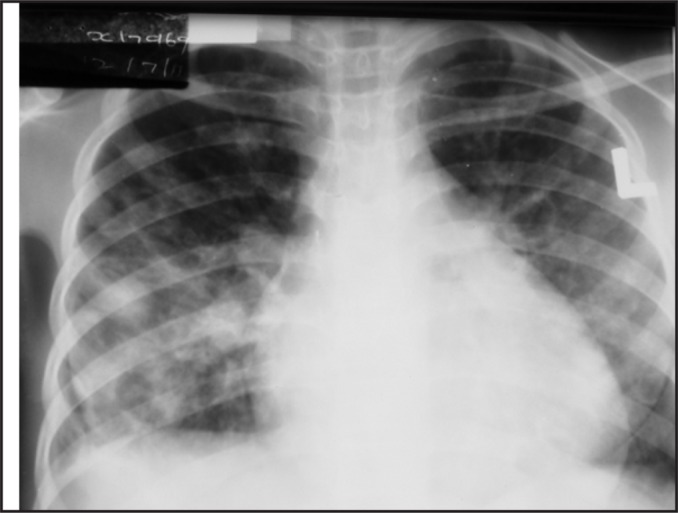
Chest x-ray - staphylococcal pneumonia
Staphylococcal pneumonia was suggested and IV cloxacillin was added to her treatments.
In view of her chest x-ray, an echocardiograph was done which showed a large oscillating intracardiac mass (vegetation) on the tricuspid valve and tricuspid valve regurgitation. There was no other cardiac abnormality, in particular no ventricular septal defect or left to right shunt producing a jet impacting on the tricuspid valve (Figures 2 and 3).
Figure 2.
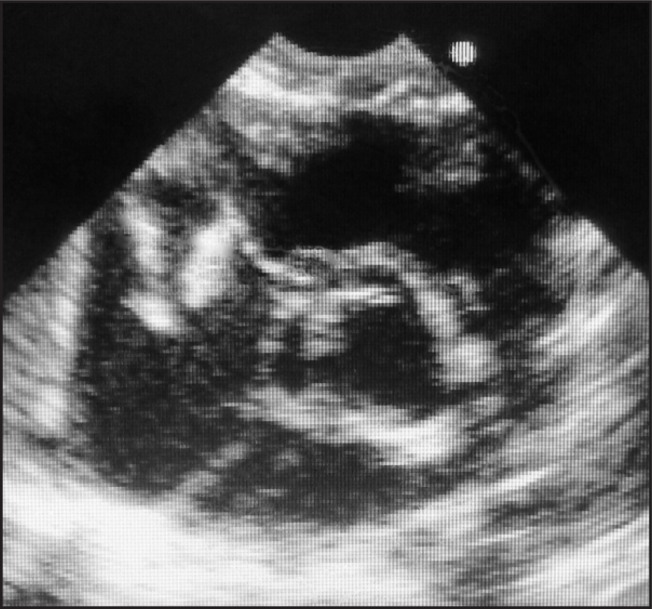
Parasternal short axis echocardiogram showing tricuspid valve vegetations (labelled ‘V’) in right atrium during systole. RA - right atrium, RV - right ventricle, A - aorta.
Figure 3.
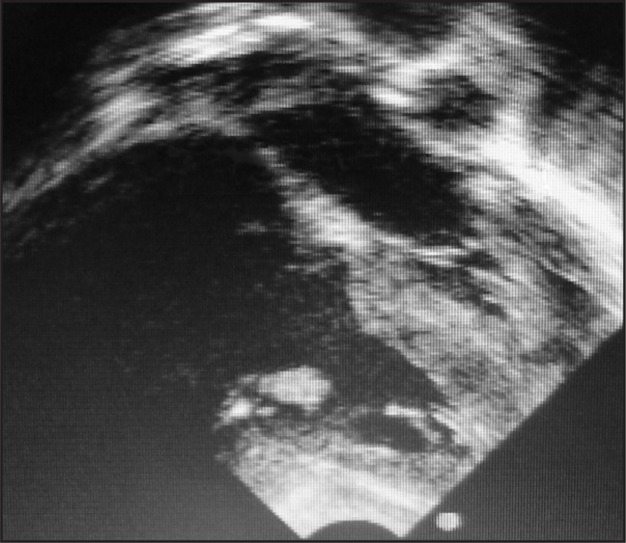
Subcostal 4 chamber echocardiogram showing tricuspid valve vegetations (labelled ‘V’) in right ventricle during diastole. RA - right atrium, RV - right ventricle, LA - left atrium
Her blood cultures subsequently grew Staphylococcus aureus sensitive to cloxacillin, gentamicin and erythromycin. Unfortunately, the blood culture was not repeated. Therefore, according to the modified Duke criteria (clinical criteria) for the diagnosis of infective endocarditis (1), she was classified as a possible infective endocarditis - with one major (endocardial involvement) and one minor (fever > 38°C) criteria.
Maintenance frusemide was added to her treatment. She required three further transfusions on days 5 and 10. Her full blood counts (FBCs) on days 5, 10 and 25 were as follows:
On day 14 of admission she developed a grade 2/6 holosystolic murmur best heard on the left lower sternal edge which persisted until the day of discharge (day 30). She was weaned off oxygen by day 18 when she saturated well in room air.
She remained intermittently febrile until day 26 of treatment. She was discharged home after 30 days of treatment with cloxacillin and frusemide with medications for a further two weeks. She was reviewed in the paediatric cardiac clinic 2 weeks after discharge when she had no symptoms or signs of cardiac failure. She still had a tricuspid regurgitation murmur. Echocardiography showed that the vegetations had resolved.
Discussion
Bacterial endocarditis is an elusive diagnosis. Its variable systemic manifestations often obscure the underlying presence of valvular infection. The Modified Duke Criteria are used in the diagnosis1
There are three diagnostic categories: definite, possible and rejected. A diagnosis of “definite infective endocarditis” is made using either pathologic or clinical criteria. See table above:
DEFINITION OF INFECTIVE ENDOCARDITIS ACCORDING TO THE MODIFIED DUKE CRITERIA
DEFINITE INFECTIVE ENDOCARDITIS
-
PATHOLOGICAL CRITERIA
Microorganisms demonstrated by culture or histological examination of a vegetation, a vegetation that has embolized, or an intracardiac abscess specimen; or
One major criterion and three minor criteria; or
Five minor criteria
POSSIBLE IE
One major criterion and one minor criterion; or
Three minor criteria
REJECTED
Firm alternative diagnosis explaining evidence of IE; OR
Resolution of IE syndrome with antibiotic therapy for < 4 days; or
No pathologic evidence of IE at surgery or autopsy, with antibiotic therapy for < 4 days; or
Does not meet criteria for possible IE as shown previously
DEFINITION OF MAJOR AND MINOR CLINICAL CRITERIA FOR THE DIAGNOSIS OF INFECTIVE ENDOCARDITIS
MAJOR CRITERIA
-
BLOOD CULTURE POSITIVE FOR IE
-
Typical microorganisms consistent with IE from two separate blood cultures:
Streptococcus viridans, Streptococcis bovis, HACEK group, Staphylococcus aureus, or community- acquired enterococci in the absence of a primary focus
Microorganisms consistent with IE from persistently positive blood cultures defined as follows; at least two positive blood samples drawn> 12hrs apart, or all of three or a majority of at least four separate cultures of blood (with first and last sample drawn at least 1 hr apart)
Single positive blood culture for Coxiella burnetii or antiphase-1 igG antibody titre > 1:800
-
-
EVIDENCE OF ENDOCARDIAL INVOLVEMENT
Echocardiogram positive for IE (transesophageal echo (TEE) recommended for patients with prosthetic valves, rated at least “possible IE” by clinical criteria, or complicated IE (paravalvar abscess); transthoracic echo (TTE) as first test in other patients) defined as follows:- Oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of an alternative anatomic explaination; or
- Abscess; or
- New partial dehiscence of prosthetic valve; or
- New valvular regurgitation (worsening or changing or preexixting murmur not sufficient)
MINOR CRITERIA
Predisposition, predisposing heart condition, or injection drug users
Fever, temperature >38°C
Vascular phenomena: major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial haemorrhage, conjuctival haemorrhages, and janeway lesions
Immunologic phenomena: glomerulonephritis, Osier nodes, Roth spots, and rheumatic factor
Microbiologic evidence: positive blood culture but does not meet a major criterion as noted previously* or serologic evidence or active infection with organisms consistent with IE
A common error in diagnosis is when an extra-cardiac complication of endocarditis is mistaken as the cause of all the symptoms. In this case, the complication (pneumonia) was recognized initially, but the endocarditis which had caused it had not. The consequent delay in correct evaluation may delay antibiotic therapy and decrease the probability of a successful outcome2. Our patient presented with a severe pneumonia and anaemia. She initially was labeled as a ‘respiratory’ patient and was treated as such. The echocardiographic findings of tricuspid regurgitation were unexpected and the diagnosis could easily have been missed. Tricuspid endocarditis accounts for only 5–10% of all cases of infective endocarditis3. Most cases occur in intravenous drug users4, patients who have had invasive instrumentation5, or in children with high-velocity jet streams of blood such as a ventricular septal defect or dysplastic atrioventricular valve.6 Although it is recognized that infection of the tricuspid valve does occur in children with previously normal hearts and no other known risk factors, this is thought to be extremely rare3. Symptoms arise from pneumonia or septic pulmonary emboli from dislodged valvular vegetative material7. Pulmonary manifestations are common in right sided endocarditis. This is because there is migration of septic material from the tricuspid valve into the pulmonary arteries. Septic material can be dispersed into a pulmonary vessel causing either pulmonary embolism or pneumonia8. Radiographic findings may resemble a staphylococcal pneumonia the features of which include extensive fluffy alveolar infiltrates involving a whole lobe or several lobes9. The valvular infection may go unnoticed because the cardiac murmur is absent in 45% of the patients with pulmonary complications10. In our patient, the murmur was either missed or absent in the initial stages. Staphylococcus aureus is the most common bacterial isolate and requires prolonged and aggressive antibiotic therapy. This includes treatment with semisynthetic β- lactamase- resistant penicillins for a minimum of 6 weeks. Surgery is indicated for large vegetations (>10mm). The surgical options include simple excision of the vegetation, valvectomy, tricuspid valve repair, or valve replacement. Valvectomy without reconstruction would result in frank regurgitation postoperatively. If the pulmonary pressure is normal (in most cases, the heart was previously normal), this may be tolerated for years. Valve replacement gives better long-term haemodynamic results when a repair is unlikely to be satisfactory, as in cases of large or multiple leaflet destruction10.
Conclusion
Isolated tricuspid valve endocarditis is rarely seen without intravenous drug use, invasive cardiac procedure or previous valvular disease. A high index of clinical suspicion is required to make the diagnosis. It should be considered in all patients with staphylococcal pneumonia.
Summary
A 12 years old, HIV non reactive girl developed tricuspid valve endocarditis and pneumonia caused by Staphylococcus aureus. She presented with symptoms and signs of pneumonia, anaemia and heart failure. The patient had no history of intravenous drug use nor had she been admitted to hospital prior to this illness. Echocardiography revealed no predisposing pre- existing abnormalities of the valve or heart. The underlying diagnosis could have been missed.
| On Admission | Day 5 | Day 10 | Day 25 | |
| Hb | 5.3g/dl | 5.2g/dl | 5.9g/dl | 10g/dl |
| WBC | 16×103/mm3 | 14×103/mm3 | 11×103/mm3 | 9.6×103/mm |
| Platelets | 23 ×103/mm3 | 122 ×103/mm3 | 127 ×103/mm3 | 300 ×103/mm |
Footnotes
Excludes single positive cultures for coagulase-negative staphylococci and organisms that do not cause endocarditis.
References
- 1.Baddour LM, Wilson WR, Bayer As, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications; a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardilology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association. Circulation. 2005;111(23):e394–e433. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 2.Chan P, Ogilby J, Segal B. Tricuspid valve endocarditis. Am Heart J. 1989;117:1140–1146. doi: 10.1016/0002-8703(89)90874-0. [DOI] [PubMed] [Google Scholar]
- 3.Swiston John, Shafran Stephen D, Kassam Norman. Isolated native tricuspid valve endocarditis caused by Streptococcus viridans. Can J Infect Dis. 2001;12(5):305–307. doi: 10.1155/2001/912750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remetz MS, Quagliarello V. Endovascular infections arising from the right sided heart. Cardiolo Clin. 1992 Feb;10(1):137–149. [PubMed] [Google Scholar]
- 5.Baddour LM, Wilson LM. Infections of prosthetic valves and other cardiovascular devices: intravascular devices. In: Mandell GL, Bennett JE, Dolin R, editors. Mandel, Douglas and Bennett's Principles and Practice and Infectious Diseases. 5th ed. Philadelphia, Pa: Elsevier; 2005. pp. 1022–2044. [Google Scholar]
- 6.Fillippo Sd, et al. Current patterns of infective endocarditis in congenital heart disease. Heart. 2006;92:1490–1495. doi: 10.1136/hrt.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penidis IP, Kotler MN, Mints GS, Segal BL, Ross JJ. Right heart endocarditis: Clinical and echocardiagraphic features. Am Heart J. 1984 Aug;69(814):615–620. doi: 10.1016/0002-8703(84)90325-9. [DOI] [PubMed] [Google Scholar]
- 8.Heffner John E. Extracardiac manifestations of bacterial endocarditis. West J Med. 1979 Aug;131(2):85–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Garvey GJ, Neu HC. Infective endocarditis- An evolving disease. Medicine (Baltimore) 1978;57(2):105–127. [PubMed] [Google Scholar]
- 10.Caleb Michael, Shankar Sriram, Agasthian Thirugnanam. Tricuspid Endocarditis and paradoxical embolism. Asian Cardiovasc Thorac Ann. 2001;9:132–134. [Google Scholar]


