Figure 3.
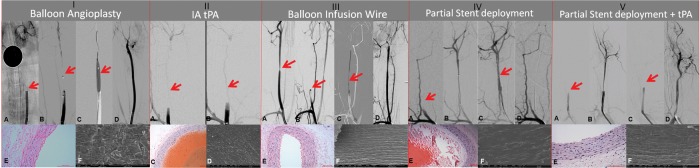
Representative results of device efficacy and safety evaluation. (I) Balloon angioplasty: a stable occlusion is confirmed on digital subtraction angiography (DSA) post clot injection and suture removal (arrow, A). A compliant balloon is positioned at the occlusion site (arrow, B) and balloon angioplasty is performed (arrow, C). Post treatment a final revascularization score of Thrombolysis in Cerebral Infarction (TICI) 2A is achieved (D). (II) Intra-arterial tissue plasminogen activator (tPA) when administered at the occlusion site (A, arrow) at a dose of 2 mg fails to revascularize the vessel (B, TICI 0). (III) Balloon infusion wire: a stenosis is created in the right common carotid artery (arrow, A). Post clot injection and suture removal reveals a stable occlusion (arrow, B). Post angioplasty and infusion of tPA through the balloon infusion wire (arrow, C) and a final revascularization score of TICI 2B is achieved (D). (IV, V) Partial stent deployment without/with tPA reveals post clot injection and suture removal, a stable occlusion (arrow, IV A,V A). Microcatheter is placed beyond the clot (IV B, V B) and the stent is deployed. There is transient restoration of flow with partial stent deployment alone (IV C), however on resheathing the vessel reoccluded (IV D). In (V), the vessel initially occluded after resheathing the stent (V C). However, secondary to the intra-arterial administration of 4 mg tPA, the clot lysed and a final revascularization score of TICI 2A is achieved (V D). Histology and scanning electron microscopy (I, III, IV, V: E and F; II: C and D), respectively, reveal the vascular safety of the treatment. In all cases there is denudation of the endothelium and exposure of an intact internal elastic lamina. (II C) and (IV E) reveal an intra-luminal thrombus.
