Fig 4.
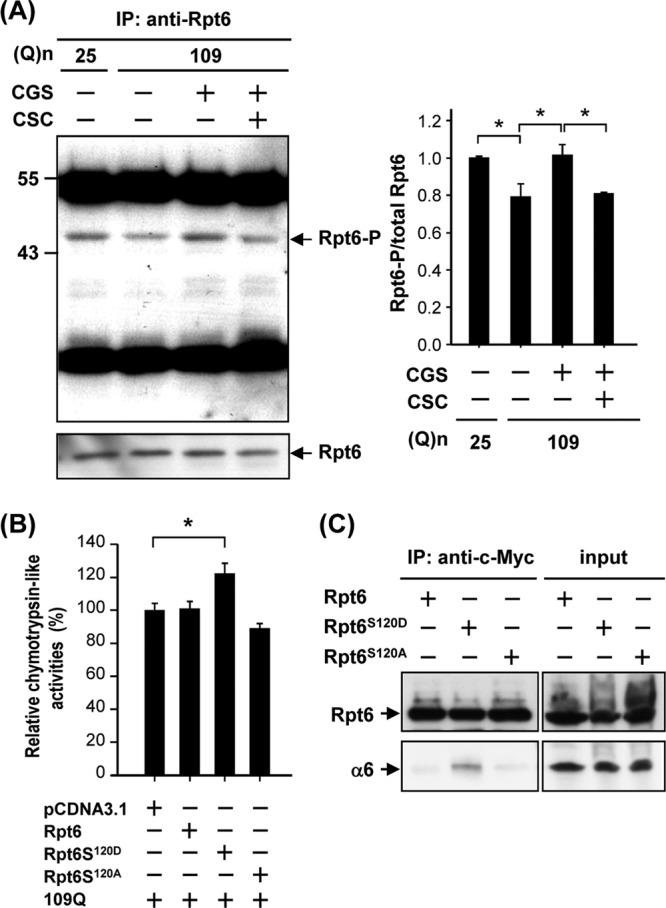
Activation of PKA via the A2A adenosine receptor (A2AR) increased phosphorylation of Rpt6 in ST14A cells. ST14A cells were transfected with pcDNA3.1-Htt-(Q)25-hrGFP or pcDNA3.1-Htt-(Q)109-hrGFP for 24 h and then treated with the desired reagent(s) for another 48 h. Rpt6 was immunoprecipitated using an anti-Rpt6 antibody, and the immunoprecipitated complexes were analyzed by Western blotting analyses, using an anti-phospho-(Ser/Thr) PKA substrate antibody and anti-Rpt6 antibody. The level of phosphorylated Rpt6 was quantified and is shown in the right panel, and Rpt6 was used as an internal control. Data are presented as the means ± SE for 3 experiments. ∗, P < 0.05 (by one-way ANOVA). (B) ST14A cells were transfected with the indicated constructs for 72 h. Chymotrypsin-like activity of proteasomes was assessed as described in Materials and Methods. (C) ST14A cells were transfected with the indicated construct for 72 h. Cell lysates were immunoprecipitated by an anti-c-Myc antibody conjugated with protein G beads. The resultant complexes were subjected to a Western blot analysis using anti-c-Myc and anti-α6 antibodies. Representative images of 3 independent experiments are shown.
