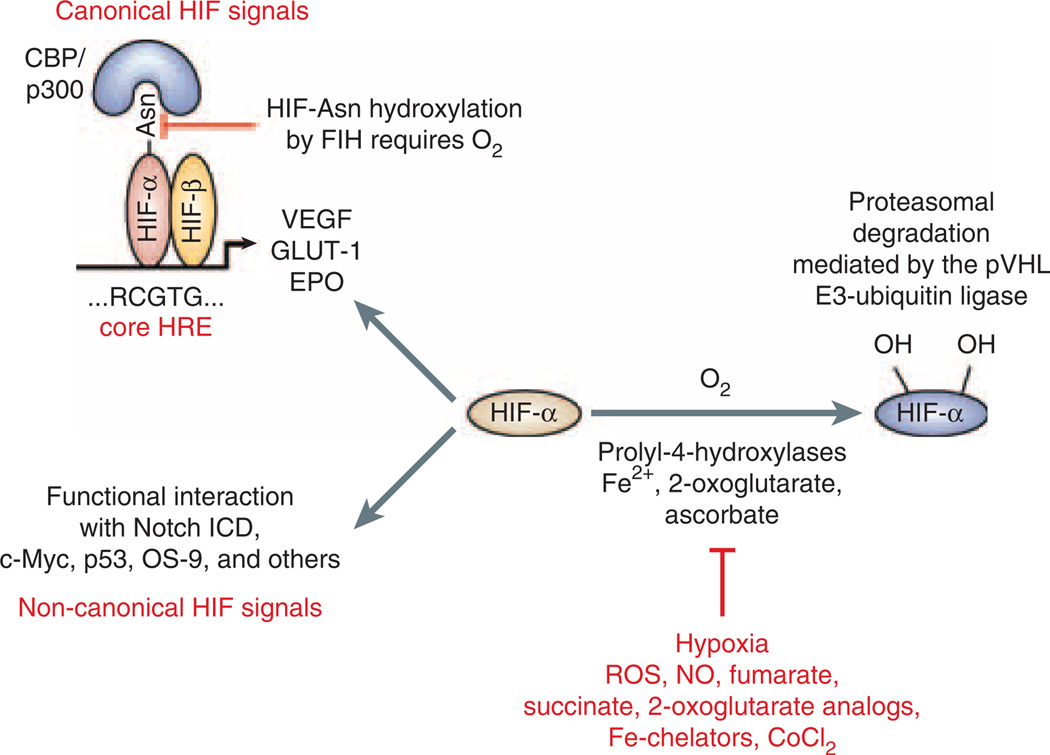
Figure 1. Overview of HIF regulation.
Under normoxia hydroxylation of hypoxia-inducible factor (HIF)-α subunits by prolyl-4-hydroxylases is required for binding to the pVHL-E3-ubiquitin ligase complex, which poly-ubiquitinates HIF-α and targets it for proteasomal degradation. When prolyl-4-hydroxylation is inhibited HIFα is stabilized and translocates to the nucleus where it heterodimerizes with HIF-β. HIF-α/β heterodimers bind to the HIF consensus binding site RCGTG and regulate transcription of HIF target genes, for example, EPO, VEGF, and GLUT-1. Factor-inhibiting-HIF (FIH) is an asparagines hydroxylase that modulates cofactor recruitment to the HIF transcriptional complex through oxygen-dependent asparagines hydroxylation of the HIF-α carboxy-terminal transactivation domain. FIH also modulates the cross talk between Notch and HIF pathways through Notch ICD hydroxylation.34 Non-canonical HIF signaling occurs through functional interaction with other proteins, such as the Notch intracellular domain (for a more complete overview of HIF-α-interacting proteins see Wenger et al.12). Nitric oxide, reactive oxygen species, the Krebs cycle metabolites succinate and fumarate, cobalt chloride, and iron chelators such as desferrioxamine are known to inhibit HIF prolyl-4-hydroxylases in the presence of oxygen. Asn, asparagine; CoCl2, cobalt chloride; EPO, erythropoietin; Fe2+, ferrous iron; GLUT-1, glucose transporter 1; HRE, hypoxia response element; NO, nitric oxide; Notch ICD, Notch intracellular domain; VEGF, vascular endothelial growth factor; ROS, reactive oxygen species.