Abstract
The pharmacokinetics of intravenous anidulafungin in adult intensive care unit (ICU) patients were assessed in this study and compared with historical data from a general patient population and healthy subjects. Intensive plasma sampling was performed over a dosing interval at steady state from 21 ICU patients with candidemia/invasive candidiasis. All patients received the recommended dosing regimen (a 200-mg loading dose on day 1, followed by a daily 100-mg maintenance dose), except for a 54-year-old 240-kg female patient (who received a daily 150-mg maintenance dose instead). Plasma samples were assayed for anidulafungin using a validated liquid chromatography-tandem mass spectrometry method. Pharmacokinetic parameters in ICU patients were calculated by a noncompartmental method. With the exclusion of the 240-kg patient, the median (minimum, maximum) age, weight, and body mass index (BMI) of 20 ICU patients were 57 (39, 78) years, 65 (48, 106) kg, and 23.3 (16.2, 33.8) kg/m2, respectively. The average anidulafungin area under the curve over the 24-hour dosing interval (AUC0-24), maximum concentration (Cmax), and clearance (CL) in 20 ICU patients were 92.7 mg · h/liter, 7.7 mg/liter, and 1.3 liters/h, respectively. The exposure in the 240-kg patient at a daily 150-mg dose was within the range observed in ICU patients overall. The average AUC0-24 and Cmax in the general patient population and healthy subjects were 110.3 and 105.9 mg · h/liter and 7.2 and 7.0 mg/liter, respectively. The pharmacokinetics of anidulafungin in ICU patients appeared to be comparable to those in the general patient population and healthy subjects at the same dosing regimen.
INTRODUCTION
Invasive candidiasis, including candidemia (ICC), is common in the intensive care unit (ICU) and accounts for up to 15 to 30% of all nosocomial infections in ICU patients (1). In a recent prevalence study, a candidemia incidence of 6.9 per 1,000 ICU patients was reported (2). ICC infections are associated with mortality rates of 30 to 50% in ICU patients (1, 2). Patients admitted to ICUs are more likely to have renal/hepatic impairment and other underlying comorbidities and to use multiple concomitant medications; thus, these patients might have poorer treatment outcomes than non-ICU patients (3–5). Because of these special conditions in ICU patients, a frequently asked question is whether the pharmacokinetics of a drug in ICU patients are significantly different from those in a general patient population, which might require a different dose recommendation.
The echinocandin antifungals, including anidulafungin, caspofungin, and micafungin, are currently recommended for the first-line treatment of ICC in moderately severe to severely ill patients and those with prior azole exposure, as well as for invasive infections due to Candida krusei or Candida glabrata (6–8). Although the three echinocandins are from the same class, differences in the route of metabolism, the requirement for a loading dose, dose adjustments in patients with moderate-to-severe hepatic disease, and the dosing schedules for different types of Candida infections exist (9). As no dose adjustment of anidulafungin is required for patients with renal/hepatic impairment and no clinically relevant drug-drug interactions are known (10, 11), anidulafungin might be a particularly valuable treatment option in ICU patients. So far, no studies have formally evaluated the pharmacokinetic profile of anidulafungin in critically ill patients.
Since ICU patients are one of the major target populations for the echinocandin class of antifungals in clinical practice, a prospective open-label multicenter phase 3b study was conducted to evaluate the efficacy, safety, and pharmacokinetics of anidulafungin in the treatment of ICC in selected adult ICU patient populations (described in “Study population” below) (12). Anidulafungin has been shown to be safe and effective as the first-line treatment of ICC in these ICU patients (12). The evaluation of the pharmacokinetics of intravenous (i.v.) anidulafungin in a subset of the ICU patients is presented here. In addition, the pharmacokinetic results from ICU patients are compared with historical data from a general patient population and healthy subjects.
(Part of this research was presented at the 5th Trends in Medical Mycology [TIMM-5], Valencia, Spain, 2 to 5 October 2011.)
MATERIALS AND METHODS
Study design.
This was an open-label, noncomparative, multicenter study in adult ICU patients with ICC. All patients were randomized to receive i.v. anidulafungin (a 200-mg loading dose on day 1, followed by a daily 100-mg maintenance dose) for 10 to 42 days. The recommended infusion rate was not to exceed 1.1 mg/min (approximately 3 h for administration of the 200-mg loading dose and 1.5 h for administration of the 100-mg maintenance dose). Patients who completed ≥10 days of i.v. treatment with anidulafungin could be switched to oral voriconazole or fluconazole on any day after day 10, provided that specific criteria were met to confirm resolution of the acute fungal infection. Intensive pharmacokinetic sampling was performed in a subset of ICU patients at 8 selected study centers in Europe (Austria, Belgium, Germany, Greece, Netherlands, and Portugal). This study was approved by the local ethics committees. Written informed consent was obtained from all patients or their legally authorized representatives prior to entering the study.
Study population.
A detailed description of the study population was presented in a previously published article (12). Briefly, the key criteria included adult ICU patients from ≥1 specific subpopulation (ie, those who had abdominal surgery, had a solid tumor, had renal insufficiency, had hepatic insufficiency, had solid organ transplantation, had neutropenia [neutrophil count, <500/mm3], or were aged ≥65 years) who had signs and symptoms of acute fungal infection within 48 h prior to initiation of the study treatment, had confirmed ICC within 96 h before or 48 h after initiation of the study treatment, and had an acute physiology and chronic health evaluation II (APACHE II) score of <25. The presence of renal and hepatic insufficiency was determined by the investigator according to local guidelines.
Intensive pharmacokinetic sampling.
All blood samples (approximately 4 ml each) were to be collected after at least 2 days of dosing with anidulafungin (i.e., from day 3 onward): at predose (prior to start of the infusion) and at 1.5, 2, 3, 4, 8, 12, and 24 h after the start of anidulafungin infusion. Plasma samples were stored at approximately −70°C until analysis.
Analytical method.
Pharmacokinetic samples were analyzed for anidulafungin concentrations at PPD (Richmond, VA) using a validated high-performance liquid chromatography-tandem mass spectrometric (LC-MS/MS) method (13). Briefly, plasma aliquots (50 μl) were diluted with 300 μl of 100 ng/ml internal standard working solution (stable-isotope-labeled [2H]11anidulafungin in methanol) or 300 μl of methanol for blanks. Analytes were isolated through protein precipitation, and a 200-μl portion of the supernatant was diluted with 600 μl of 50 mM ammonium acetate (pH 4.0), followed by centrifugation. A 10-μl final extract was injected into an LC-MS/MS system (HP 1100 series pump interfaced with a Sciex API 4000 triple-quadrupole mass spectrometer using negative-ion electrospray ionization) set up with a BDS Hypersil C8 column (2.1 by 100 mm, 5 μm; Thermo). The mobile phases used were 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B) at a flow rate of 0.1 to 0.5 ml/min. The transition ions m/z 1,138.6→898.5 (anidulafungin) and m/z 1,149.6→909.7 (internal standard) were monitored.
The dynamic range of the assay for anidulafungin was 0.05 to 20 μg/ml. The between-day assay accuracy of the quality control samples used during sample analysis ranged from −0.067% to 4.15%, with a precision of ≤7.52% for anidulafungin. All the samples were analyzed within an established long-term stability period.
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic analysis was performed using an internally validated system, eNCA v2.2.1. The maximum observed plasma concentration (Cmax), time to reach Cmax (Tmax), and trough concentration (Cmin) for anidulafungin were estimated directly from concentration-time data. The area under the concentration-time curve over the 24-h dosing interval (AUC0–24) for anidulafungin was estimated using the linear/log-trapezoidal approximation. Clearance (CL) was estimated as dose/AUC0–24. The volume of distribution at steady state (Vss) was estimated as CL × mean residence time (MRT). Samples below the lower limit of quantification were set to 0 ng/ml for analysis. Actual sample collection times were used for the pharmacokinetic analysis.
Statistical analysis.
Anidulafungin pharmacokinetic parameters were summarized with descriptive statistics. The arithmetic mean and percent coefficient of variation (%CV) are reported for all parameters except for Tmax, for which the median (range) is reported. Anidulafungin total exposure (AUC0–24) was evaluated by serum albumin concentration, since albumin concentration has been identified as a prognostic factor for caspofungin exposure in ICU patients; patients with high albumin concentrations (e.g., >23.6 g/liter) were predicted to have higher exposure to caspofungin (14). The pharmacokinetic parameters from ICU patients were compared with historical data from the general patient population, which included data from 262 patients with fungal infections in 4 phase 2/3 studies based on the population pharmacokinetic modeling approach (15). Given the imbalance of the sample size between the ICU patients in this study and the general patient population, no formal statistical analysis was performed, and the total exposures (AUC0-24) of these two groups were compared by visual inspection. If there was a substantial overlap between the distribution of the AUC0-24 in ICU patients and that in the general patient population, and if the median AUC0-24 in ICU patients was similar to that in the general patient population, the anidulafungin exposures would be considered comparable. It is known that the pharmacokinetics of anidulafungin in patients and healthy subjects are similar (16). For completeness, the historical data from healthy subjects (17) were included for cross-comparison.
RESULTS
Patients.
Intensive plasma sampling was performed at steady state (on days 3 to 8) from 21 ICU patients. All patients except one received the recommended maintenance dosing regimen (100 mg daily). The exception was a 54-year-old female morbidly obese patient (weight, 240 kg; body mass index [BMI], 83 kg/m2). Given her unusually high body weight, a higher anidulafungin maintenance dose of 150 mg daily was given to the patient at the discretion of the principal investigator in order to ensure sufficient exposure to anidulafungin, which was agreed to by the sponsor. This patient was therefore excluded from the summary statistics for the pharmacokinetic parameters, and her data are presented separately. Baseline demographics for the patients (n = 20) who received the standard dose of anidulafungin are presented in Table 1.
Table 1.
Baseline demographics of ICU patients included for pharmacokinetic assessments
| Demographic | Values for ICU patientsa (n = 20) |
|---|---|
| Sex | |
| Male (n [%]) | 11 (55) |
| Female (n [%]) | 9 (45) |
| Median age (range) (yr) | 57 (39–78) |
| Race | |
| White (n [%]) | 20 (100) |
| Mean weight (range) (kg) | 65 (48–106) |
| Mean BMI (range) (kg/m2) | 23.3 (16.2–33.8) |
One 54-year-old female patient (BMI, 83 kg/m2; weight, 240 kg) was excluded.
Anidulafungin pharmacokinetics in ICU patients compared with those for the general patient population and healthy subjects.
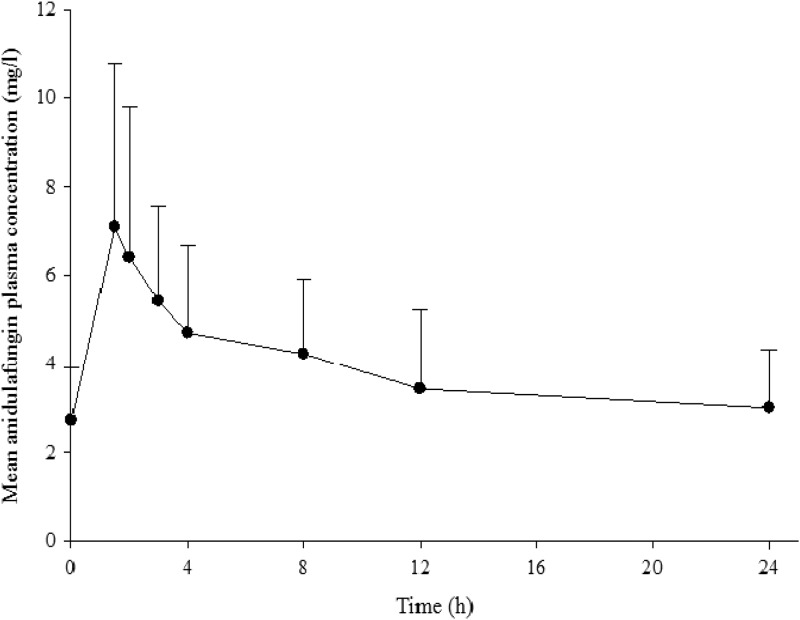
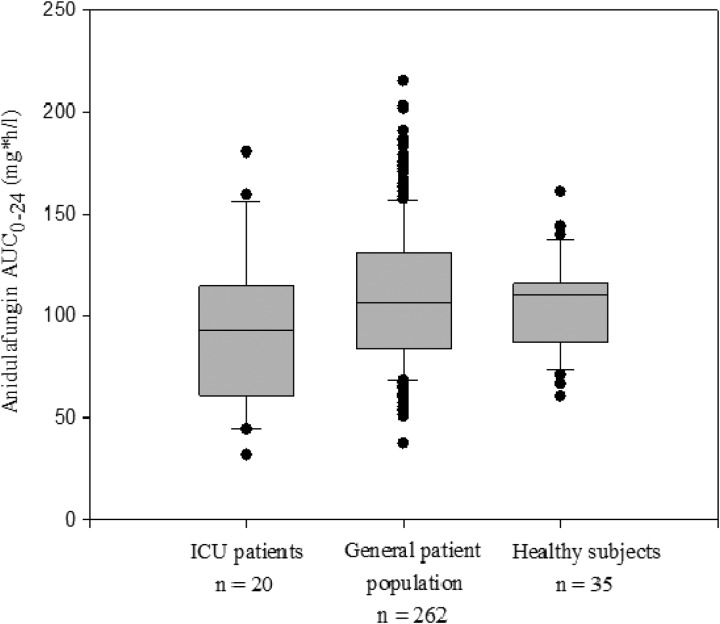
The mean anidulafungin plasma concentration-time profile in ICU patients receiving the daily 100-mg i.v. dose is presented in Fig. 1. The pharmacokinetic parameters in ICU patients, the general patient population, and healthy subjects at the same dosing regimen are summarized in Table 2. Larger intersubject variability in anidulafungin exposures was observed in ICU patients. Nonetheless, the anidulafungin total exposures in ICU patients appeared to be comparable to those in the general patient population and healthy subjects, as demonstrated by the substantial overlap of the distributions of individual AUC0–24 values between these groups (Fig. 2). In the ICU patients, the AUC0–24 ranged from 31.4 to 180 mg · h/liter, the Cmax ranged from 2.9 to 19.2 mg/liter, and CL ranged from 0.6 to 3.2 liters/h.
Fig 1.
Mean (+ standard deviation) anidulafungin plasma concentration-time profile for ICU patients receiving 100 mg i.v. anidulafungin daily (n = 20).
Table 2.
Comparison of pharmacokinetic parameters of anidulafungin in ICU patients with those of the general patient population and healthy subjects at a 200/100-mg (loading dose/maintenance dose) dosing regimen
| Pharmacokinetic parametera | Arithmetic mean (%CV)b for: |
||
|---|---|---|---|
| ICU patients (n = 20) | General patient population (n = 262)c | Healthy subjects (n = 35)d | |
| AUC0–24 (mg · h/liter) | 92.7 (41) | 110.3 (32.5) | 105.9 (21.6) |
| Cmax (mg/liter) | 7.7 (56) | 7.2 (23.3) | 7.0 (21.6) |
| Cmin (mg/liter) | 3.0 (44) | 3.3 (41.8) | 3.1 (25.0) |
| Tmax (h)e | 1.75 (1.5–4) | 1.67 | 1.70 (1.64–8) |
| CL (liters/h) | 1.3 (51) | 1.0 (33.5) | 1.0 (24.1) |
| Vss (liters) | 38.8 (51)f | 34.5 (10.5) | 35.2 (40.9) |
AUC0–24, area under the curve over 24-hour dosing interval; Cmax, maximum concentration; Cmin, minimum concentration (trough); Tmax, time to reach Cmax; CL, clearance; Vss, volume of distribution at steady state.
Percent coefficient of variation.
The parameters were estimated by population pharmacokinetic modeling (a two-compartment model with first-order elimination) using individual pharmacokinetic parameters and an infusion rate of 1 mg/min to administer anidulafungin.
The parameters were estimated by a standard noncompartmental analysis method. Data are on file for all arithmetic mean and %CV values.
Data for Tmax are median (range).
n = 10.
Fig 2.
Comparison of anidulafungin AUC0–24 in ICU patients with that in the general patient population and healthy subjects at a 200/100-mg (loading dose/maintenance dose) dosing regimen. The box plot provides medians with 10th, 25th, 75th, and 90th percentiles; values outside the 10th to 90th percentiles are represented as filled circles.
Of note, one patient weighing 106 kg (a 53-year-old male with a BMI of 33.8 kg/m2) had an inconsistent concentration-time profile (e.g., the concentrations at 4 and 8 h postdose were higher than that at 3 h postdose). Although the total exposure (AUC0–24, 31.4 mg · h/liter, the lowest value) calculated based on the observed data for this patient was questionable, it was still included in the summary statistics of this analysis. In addition, as this patient did not have a positive culture for Candida infection, he was discontinued from the study on day 4 due to a failure to meet enrollment criteria; thus, efficacy endpoints were not assessed in this patient.
Anidulafungin pharmacokinetics in a morbidly obese ICU patient.
For the 240-kg female patient (BMI, 83 kg/m2) who received the higher dose of 150 mg i.v. daily, the AUC0–24 (55.3 mg · h/liter) fell within the range observed for ICU patients who received a standard dose of 100 mg i.v. daily. In this obese patient, the Cmax, Tmax, and CL also fell within the ranges observed in this study overall (Cmax = 3.1 mg/liter, Tmax = 1.8 h, and CL = 2.7 liters/h). It was noted that this patient had global success (clinical cure and microbiological eradication) at the end of treatment. If this patient had received the 100-mg dose, the total exposure (AUC0–24) would have been approximately 37 mg · h/liter by extrapolation based on the linear pharmacokinetic properties of anidulafungin.
Correlation between anidulafungin AUC0–24 and serum albumin concentrations.
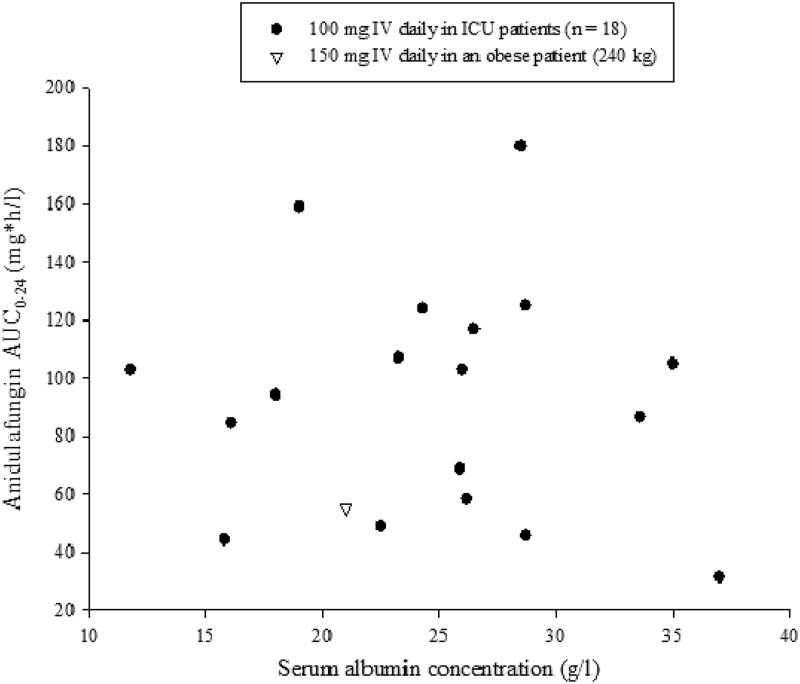
Except for two patients, albumin data were available on the day of pharmacokinetic sampling or within 3 days of the sampling date for all patients. No trend was observed between individual anidulafungin AUC0–24 values and serum albumin concentrations in these ICU patients (Fig. 3).
Fig 3.
Individual anidulafungin AUC0–24 in ICU patients by serum albumin concentrations.
DISCUSSION
The findings from this study show that the physiological changes in ICU patients did not appear to have a significant impact on the disposition of anidulafungin compared with those in typical patient populations and healthy subjects. This was expected based on the pharmacokinetic characteristics of anidulafungin. It is known that anidulafungin undergoes slow chemical degradation to produce a ring-opened peptide that lacks antifungal activity, which is subsequently converted to peptidic degradants and eliminated in feces. Anidulafungin has negligible renal clearance (<1%). Anidulafungin is not a clinically relevant substrate, inducer, or inhibitor of cytochrome P450 enzymes.
The observed intersubject variability in total exposure (AUC0–24) was higher in ICU patients than in the general patient population and healthy subjects (i.e., 41% vs 32.5% vs 21.6%, respectively) (Table 2). This is probably attributable to the serious underlying conditions of the ICU patients (e.g., sepsis, septic shock, surgery), which might have a slight impact on drug disposition.
Nguyen et al. reported that caspofungin trough concentrations in surgical ICU patients were slightly higher than those in healthy subjects, and the intersubject variability was higher (14). Another study showed the opposite trend: caspofungin exposures (AUC0–24) in surgical ICU patients were lower than those in a general patient population (18). Nonetheless, both studies suggest that low serum albumin concentrations in ICU patients are correlated with low caspofungin exposures. However, our study did not show any correlation between serum albumin concentrations and anidulafungin exposures (Fig. 3). Given the limited sample size in our study, further investigation on this relationship is warranted.
It is known that physiological changes caused by obesity can significantly alter a drug's disposition (e.g., distribution, metabolism, and elimination) (19). Dosing in morbidly obese patients is a challenge that is frequently encountered in clinical practice. Based on the simulation of anidulafungin exposure and the exposure-efficacy analysis in patients with high body weight, we demonstrated that patients weighing up to 150 kg can receive the standard dosing regimen (100 mg daily) without compromising efficacy (15). Our study did not specifically focus on obese patients. The anecdotal findings reported here for a single morbidly obese patient suggest that increasing the anidulafungin dose in patients weighing ≥200 kg or with a BMI of ≥80 kg/m2 might be required (e.g., 50% increase of the standard dose); however, further evaluation will be needed in order to determine optimal dosing strategies.
In summary, the pharmacokinetics of i.v. anidulafungin in ICU patients appeared to be comparable to those in the general patient population and healthy subjects at the same dosing regimen.
ACKNOWLEDGMENTS
This study was sponsored by Pfizer Inc.
We sincerely thank all the clinical staff from 8 study centers for their outstanding support to this study (University Hospital Leuven, Leuven, Belgium; Hospital de São João, Porto, Portugal; Charité Universitätsmedizin, Berlin, Germany; Cliniques Universitaires UCL de Mont-Godinne Yvoir, Belgium; Hopital Universitaire Erasme, Brussels, Belgium; Ziekenhuis Gelderse Vallei, Ede, the Netherlands; General Hospital of Attica, Athens, Greece; and University Clinics–General Hospital Vienna, Vienna, Austria). We thank our study team at Pfizer, who contributed to the protocol and study. We also thank Christine Alvey (Pfizer) for the noncompartmental data analysis and PPD (Richmond, VA) for the analytical assay support.
P.L., M.K., and B.D. are employees of Pfizer, and M.R., W.M., and J.A.P. were the principal investigators for this study.
Footnotes
Published ahead of print 18 January 2013
REFERENCES
- 1. Vazquez JA. 2010. Invasive fungal infections in the intensive care unit. Semin. Respir. Crit. Care Med. 31:79–86 [DOI] [PubMed] [Google Scholar]
- 2. Kett DH, Azoulay E, Echeverria PM, Vincent JL, Extended Prevalence of Infection in the ICU Study (EPIC II) Group of Investigators. 2011. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit. Care Med. 39:665–670 [DOI] [PubMed] [Google Scholar]
- 3. DiNubile MJ, Lupinacci RJ, Strohmaier KM, Sable CA, Kartsonis NA. 2007. Invasive candidiasis treated in the intensive care unit: observations from a randomized clinical trial. J. Crit. Care 22:237–244 [DOI] [PubMed] [Google Scholar]
- 4. Dupont BF, Lortholary O, Ostrosky-Zeichner L, Stucker F, Yeldandi V. 2009. Treatment of candidemia and invasive candidiasis in the intensive care unit: post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit. Care 13:R159 doi:10.1186/cc8117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kett DH, Shorr AF, Reboli AC, Reisman AL, Biswas P, Schlamm HT. 2011. Anidulafungin compared with fluconazole in severely ill patients with candidemia and other forms of invasive candidiasis: support for the 2009 IDSA treatment guidelines for candidiasis. Crit. Care 15:R253 doi:10.1186/cc10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guery BP, Arendrup MC, Auzinger G, Azoulay E, Borges Sa M, Johnson EM, Muller E, Putensen C, Rotstein C, Sganga G, Venditti M, Zaragoza Crespo R, Kullberg BJ. 2009. Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: part I. Epidemiology and diagnosis. Intensive Care Med. 35:55–62 [DOI] [PubMed] [Google Scholar]
- 7. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group (EFISG) 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin. Microbiol. Infect. 18:19–37 [DOI] [PubMed] [Google Scholar]
- 9. Kofla G, Ruhnke M. 2011. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur. J. Med. Res. 16:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowell JA, Stogniew M, Krause D, Damle B. 2007. Anidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairment. J. Clin. Pharmacol. 47:461–470 [DOI] [PubMed] [Google Scholar]
- 11. Damle BD, Dowell JA, Walsky RL, Weber GL, Stogniew M, Inskeep PB. 2009. In vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafungin. Antimicrob. Agents Chemother. 53:1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruhnke M, Paiva JA, Meersseman W, Pachl J, Grigoras I, Sganga G, Menichetti F, Montravers P, Auzinger G, Dimopoulos G, Sa MB, Miller PJ, Marcek T, Kantecki M. 2012. Anidulafungin for the treatment of candidaemia/invasive candidiasis in selected critically ill patients. Clin. Microbiol. Infect. 18:680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alebic-Kolbah T, Modesitt MS. 2012. Anidulafungin-challenges in development and validation of an LC-MS/MS bioanalytical method validated for regulated clinical studies. Anal. Bioanal. Chem. 404:2043–2055 [DOI] [PubMed] [Google Scholar]
- 14. Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, Weigand MA. 2007. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J. Antimicrob. Chemother. 60:100–106 [DOI] [PubMed] [Google Scholar]
- 15. Liu P. 2013. Population pharmacokinetic-pharmacodynamic analysis of anidulafungin in adult patients with fungal infections. Antimicrob. Agents Chemother. 57:466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfizer 2010. Eraxis (anidulafungin) package insert. Pfizer, New York, NY [Google Scholar]
- 17. Dowell JA, Stogniew M, Krause D, Henkel T, Damle B. 2007. Lack of pharmacokinetic interaction between anidulafungin and tacrolimus. J. Clin. Pharmacol. 47:305–314 [DOI] [PubMed] [Google Scholar]
- 18. Pascual AA, Senn L, Bolay S, Rochat B, Eggimann P, Ksontini R, Buclin T, Bille J, Calandra T, Marchetti O. 2007. Low total plasma concentration of caspofungin in surgical intensive care unit patients, abstr M2025, p 465 Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 19. Hanley MJ, Abernethy DR, Greenblatt DJ. 2010. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 49:71–87 [DOI] [PubMed] [Google Scholar]