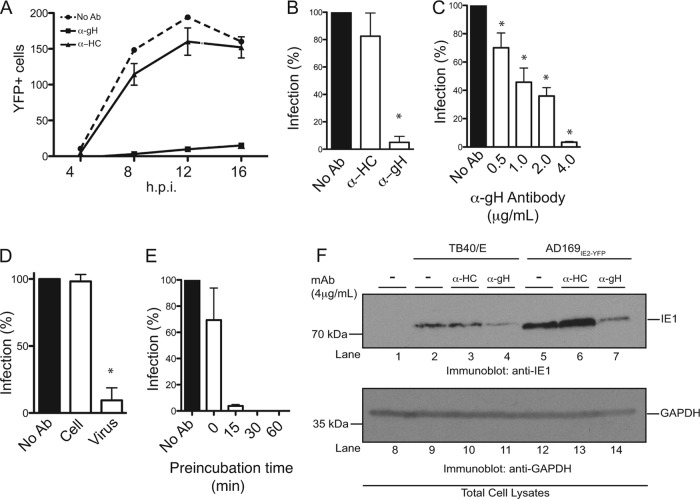
Fig 4.
Establishment of a high-throughput HCMV neutralization assay. (A) MRC5 cells infected with AD169IE2-YFP (MOI of 5) preincubated with anti-gH, anti-MHC class I heavy chain (HC), or medium were analyzed at 4, 8, 12, and 16 hpi for the number of YFP-positive cells per well by using a microplate cytometer. (B to E) The percentage of infected cells (12 hpi) was calculated by using the number of YFP-positive cells from AD169IE2-YFP-infected cells as 100%. Student's t test confirmed statistical significance (*, P < 0.05), and error bars represent standard errors of means in all experiments (B to E). (C) AD169IE2-YFP-infected cells preincubated with various concentrations (0.5 to 4.0 μg/ml) of anti-gH or anti-HC antibody or medium were analyzed (12 hpi) to determine the optimal neutralization concentration of anti-gH antibody. (D) MRC5 cells infected with AD169IE2-YFP (MOI of 5) and pretreated with anti-gH antibody or cells pretreated with anti-gH prior to infection were analyzed (12 hpi) to validate the specificity of the anti-gH antibody. (E) MRC5 cells infected with AD169IE2-YFP (MOI of 5) and pretreated with anti-gH antibody for 0, 15, 30, and 60 min were analyzed (12 hpi) to measure the minimal time required for neutralization. (F) MRC5 cells infected with TB40/E or AD169IE2-YFP (MOI of 2) preincubated with anti-gH or anti-HC antibody (4 μg/ml) were subjected to immunoblot analysis (12 hpi) for IE1 (lanes 1 to 7) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (lanes 8 to 14) proteins. The respective polypeptides and relative molecular mass markers are indicated.