Abstract
The encystation of Acanthamoeba leads to the formation of resilient cysts from vegetative trophozoites. This process is essential for parasite survival under unfavorable conditions, such as those associated with starvation, low temperatures, and biocides. Furthermore, cysteine proteases have been implicated in the massive turnover of intracellular components required for encystation. Thus, strict modulation of the activities of cysteine proteases is required to protect Acanthamoeba from intracellular damage. However, mechanisms underlying the control of protease activity during encystation have not been established in Acanthamoeba. In the present study, we identified and characterized Acanthamoeba cysteine protease inhibitor (AcStefin), which was found to be highly expressed during encystation and to be associated with lysosomes by fluorescence microscopy. Recombinant AcStefin inhibited various cysteine proteases, including human cathepsin B, human cathepsin L, and papain. Transfection with small interfering RNA against AcStefin increased cysteine protease activity during encystation and resulted in incomplete cyst formation, reduced excystation efficiency, and a significant reduction in cytoplasmic area. Taken together, these results indicate that AcStefin is involved in the modulation of cysteine proteases and that it plays an essential role during the encystation of Acanthamoeba.
INTRODUCTION
Acanthamoeba is the causative agent of granulomatous amoebic encephalitis (GAE) and amoebic keratitis. The life cycle of Acanthamoeba is divided into two stages, that is, the vegetative trophozoite stage and the dormant cystic stage. Under challenging conditions, such as those associated with starvation, low temperatures, and biocide treatment, the trophozoite converts to the resilient cyst form (1–5). This differentiation, which is termed encystation, protects Acanthamoeba against host immune responses and allows it to evade the effect of chemical treatments, and thus, encystation decreases treatment efficacies (3, 6). Therefore, the inhibition of encystation during medical treatment could lead to outcomes that are more favorable in cases of amoebic infection. However, this goal is hindered by a lack of information about the encystation mechanism involved.
The cysteine proteases are regarded as major drug targets, and the papain family of cysteine proteases, such as cathepsin B and cathepsin L, are localized in lysosomes for intracellular protein degradation (7, 8). Previous studies on Giardia and Entamoeba have shown that cysteine proteases are implicated in parasite survival under nutrient-limited conditions and during developmental differentiation (9–14). In Acanthamoeba, the cysteine protease inhibitor E64d was found to slow encystment (15), and in a previous study, we detected the upregulations of various proteases, including cysteine proteases, by cDNA microarray analysis and comparative expression analysis of expressed sequence tags (ESTs) during encystation (16, 17). Cysteine proteases in lysosomes cause massive proteolytic degradation of unnecessary cytosolic compartments or organelles during encystation and thus must be strictly controlled to protect essential intracellular components from damage for subsequent excystation of cysts to viable trophozoites.
The cystatin I25 superfamily is a well-established family of cysteine protease inhibitors, which is composed of three distinct subfamilies according to the MEROPS classification (18, 19): I25A (stefins, family 1, type 1, and cystatins A and B), I25B (cystatins, family 2, type 2, and cystatins C, E, and S), and I25C (kininogens, family 3, and type 3). The stefins (subfamily I25A) are cytoplasmic proteins with a single domain of approximately ∼11 kDa (∼100 amino acid residues) and no disulfide bonds, whereas the cystatins (subfamily I25B) are secretory proteins of 120 amino acids with two disulfide bonds and are typically found in biological fluids. On the other hand, the kininogens (subfamily I25C) are large secretory proteins containing multidomain cystatins. Most known parasite cystatins were classified as the I25 superfamily, which were identified in multicellular organisms such as nematodes, trematodes, or ticks based on evaluations of their abilities to protect parasites from extracellular proteolytic damage or to regulate intracellular proteases or based on their immunomodulatory effects (1, 20–30). In protozoan parasites, chagasin, a specific parasite-derived inhibitor of clan CA (family C1 cysteine peptidases), was identified in Trypanosoma cruzi (31). Chagasin and chagasin-like inhibitors (ICP [inhibitor of cysteine peptidases]) lack significant identity with proteins of the cystatin I25 family and are classified as chagasins (clan XI, family I42), which have been suggested to regulate endogenous and/or host cell proteases (31–42). However, little is known regarding the presence of cystatins of the I25 family in protozoan parasites or of their involvements in differentiation.
In the present study, we identified AcStefin, a cysteine protease inhibitor of the I25 family in Acanthamoeba, which was highly expressed during encystation. In addition, we examined the biochemical properties and the intracellular localization of AcStefin and investigated its roles during the encystation of Acanthamoeba.
MATERIALS AND METHODS
Cultivation of amoebae and induction of encystation and excystation.
Acanthamoeba castellanii, which was originally isolated as a eukaryotic cell culture contaminant, was obtained from the American Type Culture Collection, Manassas, VA (ATCC 30011). Amoebae were cultured axenically in peptone-yeast-glucose (PYG) medium at 25°C. Encystment was induced as previously described, with slight modifications (43). Briefly, cells from post-logarithmic-growth-phase cultures were collected aseptically. Approximately 5 × 105 cells were then washed with phosphate-buffered saline (PBS) and incubated in 10 ml of encystment medium (95 mM NaCl, 5 mM KCl, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3, 20 mM Tris-HCl [pH 9.0]) for 72 h. The morphological change of cells to cysts was observed under a light microscope. Encystation ratios were calculated by counting cysts under a light microscope after treating them with 0.05% Sarkosyl and 0.22% trypan blue, which selectively stains nonviable cells (44, 45). The same numbers of cysts from cells transfected with scrambled small interfering RNA (siRNA) or AcStefin siRNA were cultivated in nonnutrient agar covered with an emulsion of Escherichia coli at 27°C. Excystation ratios were calculated by dividing the observed number of cysts by the sum of the numbers of cells 7 days after excystation induction.
mRNA sequence of Acanthamoeba AcStefin and real-time PCR.
The full-length cDNA sequences of A. castellanii AcStefin (accession cluster ID ACL00003305) was first isolated from the taxonomically broad EST database (TBestDB) (http://tbestdb.bcm.umontreal.ca) and verified by reverse transcription-PCR (RT-PCR). Total RNA was purified by using TRIzol reagent (Gibco BRL, Rockville, MD), and cDNA synthesis was conducted by using a RevertAid first-strand cDNA synthesis kit (Fermentas, Hanover, IN). Real-time PCR was performed by using a GenAmp 5700 SDS unit (Biosystems, Barcelona, Spain), using the default thermocycler program for all genes, that is, 10 min of preincubation at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The relative expression levels of AcStefin were calculated with respect to the internal standard, Acanthamoeba actin. Individual reactions were carried out in 96-well plates containing the following designed primers: AcStefin sense primer 5′-CCTGCTGATGATGAGGTCAA-3′ and antisense primer 5′-AGGTCATCACCATCGGTAACG-3′ and Acanthamoeba actin gene (the reference gene) (GenBank accession no. CAA23399) (46) sense primer 5′-AGGTCATCACCATCGGTAACG-3′ and antisense primer 5′-TCGCACTTCATGATCGAGTTG-3′. All reactions were performed by using SYBR Premix Ex Taq (TaKaRa, Shiga, Japan).
Transient transfection.
To determine the intracellular localization of AcStefin, the AcStefin gene was cloned into the pUb vector including the Acanthamoeba ubiquitin promoter for constitutive expression and which included the gene for reporter enhanced green fluorescent protein (EGFP) (47). The AcStefin gene was PCR amplified with primers that included sites for SpeI at the 5′ end and XbaI at the 3′ end, and AcStefin was inserted into the pUb vector downstream of the EGFP gene. The amplified DNA fragment was completely sequenced (Macrogen, Seoul, South Korea). A. castellanii was then transfected with the cloned plasmid as previously described (47). Briefly, approximately 4 × 105 cells were grown to mid-log phase, washed with PBS, and resuspended in 3 ml of PYG culture medium. Aliquots were seeded into a 6-well culture plate and incubated at 25°C. Plasmid DNA (4 μg) was mixed with 20 μl of Superfect transfection reagent (Qiagen, Valencia, CA) and transferred into the cells. After incubation for 24 h, the transfected cells were placed into encystation medium and incubated for 24, 48, or 72 h. EGFP-AcStefin expression was examined by fluorescence microscopy.
Production of recombinant AcStefin and antiserum against AcStefin.
To produce recombinant AcStefin protein, its amplified PCR product (sense primer 5′-CCTGCTGATGATGAGGTCAA-3′ and antisense primer 5′-CTGAGTGGCGTACGAGACAG-3′) was cloned into the pBAD-TOPO vector (Invitrogen, Carlsbad, CA). The Escherichia coli TOP10F′ strain was then transformed with the constructed plasmid. Recombinant AcStefin protein was purified by using Ni-nitrilotriacetic acid (NTA) agarose according to the manufacturer's instructions (Qiagen), and rat antiserum against AcStefin was generated by using purified recombinant AcStefin. For immunoblot assays, cell lysates of A. castellanii trophozoites and cysts (24 h, 48 h, or 72 h after encystation induction) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred from gels onto nitrocellulose membranes. Blots were blocked and incubated with antisera collected from AcStefin-immunized rats (1/1,000 dilution) for 1 h. After washing, membranes were incubated with horseradish peroxidase-conjugated anti-rat IgG antibody (1/2,000 dilution) (Amersham Biosciences, Buckinghamshire, United Kingdom), stripped, and reprobed with mouse monoclonal anti-actin antibody (Santa Cruz Biotechnology, CA) to control for equal loading. The proteins were detected by chemiluminescence using enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences).
Inhibitory activity assay of AcStefin protein.
Inhibitory effects against the cysteine proteases human liver cathepsin L (Calbiochem, San Diego, CA), human liver cathepsin B (Calbiochem), and papain (Sigma-Aldrich, St. Louis, MO) were determined by measuring residual proteolytic activity after incubating each enzyme with recombinant AcStefin. Briefly, each cysteine protease (20 nM) was preincubated with 20 nM recombinant AcStefin protein or bovine serum albumin (BSA) (as a control) in reaction buffer (for papain, 100 mM sodium acetate [pH 6.0], 1 mM EDTA, and 2 mM dithiothreitol; for cathepsins B and L, 100 mM sodium acetate [pH 5.0] and 1 mM EDTA) for 30 min at 37°C (for papain and cathepsin B) or 25°C (for cathepsin L). Colorimetric or fluorogenic peptide substrates were then added, and the mixture was incubated for 1 h. The reaction was terminated by adding monoiodoacetate buffer to the mixture, and the absorbance was then measured by using a SpectraMax Gemini XS spectrofluorometer (Molecular Devices, Sunnyvale, CA). Substrates for the cysteine proteases were benzyloxycarbonyl–l-arginine–l-arginyl–4-nitroanilide (Z-Arg-Arg-pNA) (Calbiochem) for human cathepsin B, N-benzyloxycarbonyl–Phe–Ala–7-amino–4-trifluoromethyl coumarin (Z-Phe-Arg-AFC) (TFA) (Enzo Life Science, Farmingdale, NY) for human cathepsin L, and N-acetylphenylalanylglycine–4-nitroanilide (Ac-Phe-Gly-pNA) (Calbiochem) for papain. l-trans-3-Carboxyoxiran–2-carbonyl–l-leucylagmatine (E64) was used as a control. To calculate approximate Ki values of AcStefin against individual enzymes, different concentrations of AcStefin (0 to 100 nM) were incubated with each enzyme (20 nM), and substrate hydrolysis was monitored, as described above. Cysteine protease activities in cell extracts of scrambled-siRNA- or AcStefin-siRNA-transfected cells were determined by using the specific cathepsin L substrate Z-Phe-Arg-AFC (Enzo Life Science, Farmingdale, NY). Cell extracts (10 μg) containing reaction buffer (100 mM sodium acetate [pH 5.0], 1 mM EDTA) were preincubated for 30 min at 25°C before addition of 10 μM substrate. For controls, cell extracts of scrambled-siRNA- or AcStefin-siRNA-transfected cells were incubated with 10 nM recombinant AcStefin protein or 10 nM E64. After incubation for 1 h at 25°C, reactions were monitored as described above.
Knockdown of AcStefin.
siRNA targeting the AcStefin gene of A. castellanii was synthesized by Sigma-Proligo (Boulder, CO), based on the cDNA sequence of the gene. The siRNA oligonucleotide was designed with a fluorescein isothiocyanate (FITC) modification to allow transfection efficiency to be determined by flow cytometry. The sequence of the upper strand was 5′-GUUCCAAUCGAACGUGAGU-3′. A. castellanii trophozoites plated at a density of 4 × 105 cells were transfected with siRNA (4 μg) against AcStefin or with scrambled siRNA as a negative control (Ambion, Austin, TX), and transfection efficiencies were determined.
Immunofluorescence assay and transmission electron microscopy.
After isolating amoebae expressing EGFP-fused AcStefin by FACSAria flow cytometry (BD Biosciences, San Jose, CA), cells were allowed to adhere to culture dishes and examined by confocal laser scanning microscopy using an LSM 5 Exciter apparatus (Carl Zeiss, Hamburg, Germany). EGFP-fused AcStefin- and LysoTracker Red DND 99 (Molecular Probes, Eugene, OR)-mediated fluorescence was measured by using band-pass filters that provided excitation and emission wavelengths of 500 to 530 nm and 570 to 590 nm, respectively. For transmission electron microscopy (TEM), cell suspensions were centrifuged, and sediments were washed three times in cold PBS. The sediments were then prefixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 3 h, rinsed with 0.1 M phosphate buffer, postfixed with 1% osmium tetroxide for 2 h, rinsed twice with 0.1 M phosphate buffer, dehydrated by using an ethyl alcohol gradient (50%, 70%, 80%, 95%, and 100%), treated twice with propylene oxide resin (1:1 dilution) for 20 min, embedded in epoxy resin (Embed-812; Electron Microscopy Sciences), and incubated at 37°C for 12 h, 45°C for 12 h, and 60°C overnight. Ultrathin sections were cut on a Reichert-Jung ultramicrotome and stained with uranyl acetate and lead citrate. Sections were observed by using a model H-7000 TEM instrument (Hitachi, Tokyo, Japan). Areas of intercyst space and of cytoplasm of scrambled-siRNA- or AcStefin-siRNA-transfected cells were quantified by image analysis using ImageJ Java software (http://rsb.info.nih.gov/ij/), by calculating numbers of pixels in regions of interest. Changes in the intercyst space or cytoplasm area were determined by dividing the pixel count of scrambled-siRNA-transfected cells by the pixel count of AcStefin-siRNA-transfected cells.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper (AcStefin) has been submitted to the GenBank database with accession number JN793952.
RESULTS
Identification of the Stefin homologue in A. castellanii.
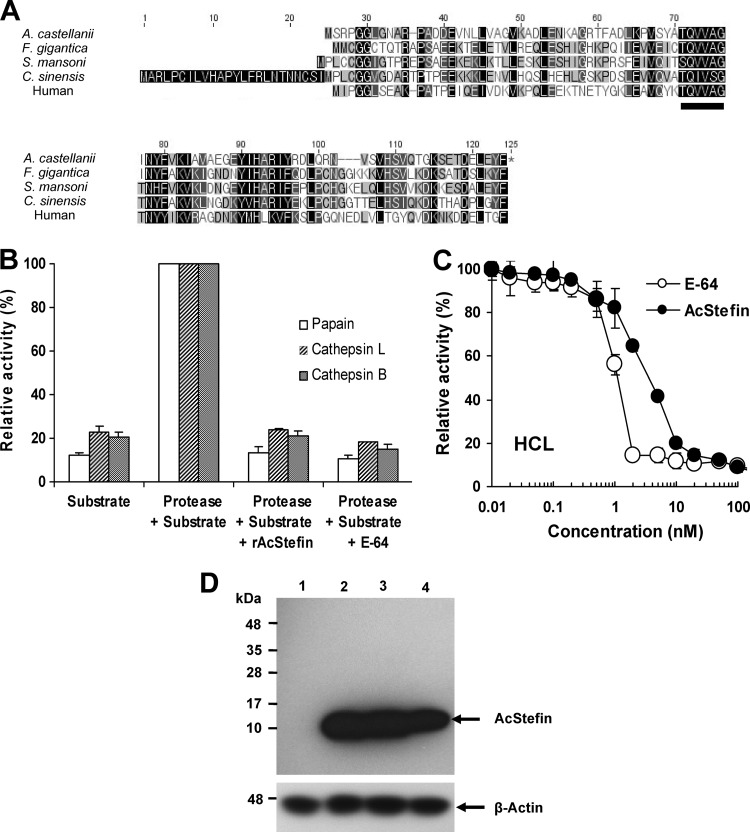
Previously, we studied the cyst-specific gene expression profile of Acanthamoeba and found that cystatin-like proteins are highly upregulated during encystation (17). Because the role of cystatin (a member of the I25 family) in encystation has not been studied in protozoan parasites, we focused on Acanthamoeba cystatin-like proteins to determine whether cystatin is involved in the encystation of Acanthamoeba. The gene showing homology with cystatin contained a 291-bp open reading frame (ORF) encoding a 96-amino-acid peptide with a calculated molecular mass of 10.5 kDa. When the deduced amino acid sequence was aligned with those of other proteins, the encoded protein exhibited strong sequence similarity with subfamily I25A (stefins) of the cystatin superfamily characterized in other organisms, such as Fasciola gigantica stefin-1 (28), Clonorchis sinensis CsStefin-1 (29), Schistosoma mansoni cystatin (2), and human cystatin A (48) (Fig. 1A). The encoded protein has a highly conserved Gln-Val-Val-Ala-Gly (QVVAG) motif in the β-hairpin loop of I25A subfamily members (stefins), which has been implicated in binding to target enzymes (49). Therefore, this sequence was designated AcStefin (Acanthamoeba castellanii stefin).
Fig 1.
Identification of AcStefin as a cysteine proteinase inhibitor in A. castellanii. (A) Sequence analysis of AcStefin. Alignments of the predicted amino acid sequences of AcStefin (top) with Fasciola gigantica stefin-1 (GenBank accession no. ASC35603) (40.4% amino acid identity), Schistosoma mansoni cysteine protease inhibitor (GenBank accession no. AAQ16180) (40.6% amino acid identity), Clonorchis sinensis (GenBank accession no. EF550966) (40.0% amino acid identity), and human cystatin A (GenBank accession no. NP_005204) (36.7% amino acid identity) were performed by using ClustalW software. Black and gray boxes indicate identical and conserved amino acids, respectively. The QVVAG cystatin motif is indicated as a bold line below the sequences. (B) Inhibitory activities of AcStefin against cysteine proteases. Inhibitory activities of recombinant AcStefin (rAcStefin) were measured by detecting the release of cleaved moieties of colorimetric or fluorometric peptide substrates after incubation with papain, human cathepsin L, or human cathepsin B. Protease activity (20 nM) only in the presence of substrates was considered 100%, and the percentages of residual activities in the presence of inhibitor (recombinant AcStefin or E64) were calculated. Data represent means ± standard deviations of triplicate samples. (C) Human cathepsin L (HCL) (20 nM) was incubated with different concentrations of recombinant AcStefin (0 to 100 nM) or E64. Residual enzyme activities were measured, and the Ki value (0.046 ± 0.0015 nM) of AcStefin against human cathepsin L was calculated. Data represent means ± standard deviations of triplicate samples. (D) Crude trophozoite extracts (lane 1) and cyst extracts at 24 h (lane 2), 48 h (lane 3), and 72 h (lane 4) after encystation of A. castellanii were resolved by SDS-PAGE and Western blotted with rat polyclonal anti-AcStefin sera (top). Membranes were reprobed with anti-actin antibody to ensure equal loading (bottom).
AcStefin is functional and highly expressed during the encystation of A. castellanii.
Next, recombinant AcStefin was expressed in E. coli to examine its biochemical properties. To ascertain the enzymatic characteristics of AcStefin, recombinant AcStefin was prepared, and its inhibitory effects against C1 family cysteine proteinase, such as human cathepsin L, human cathepsin B, and papain, were determined by using those specific peptide substrates. AcStefin was found to inhibit all enzymes tested (Fig. 1B and C), indicating that recombinant AcStefin protein is functionally active as a cysteine protease inhibitor. We then examined the expression levels of AcStefin in Acanthamoeba trophozoites and cysts using specific antibodies against the recombinant AcStefin protein. As shown in Fig. 1D, the expression of AcStefin was hardly detected in trophozoites but was significantly enhanced at 24 h postencystation and maintained at 72 h (Fig. 1D, lanes 2 to 4).
AcStefin localizes to lysosomes/autophagolysosomes in Acanthamoeba cysts.
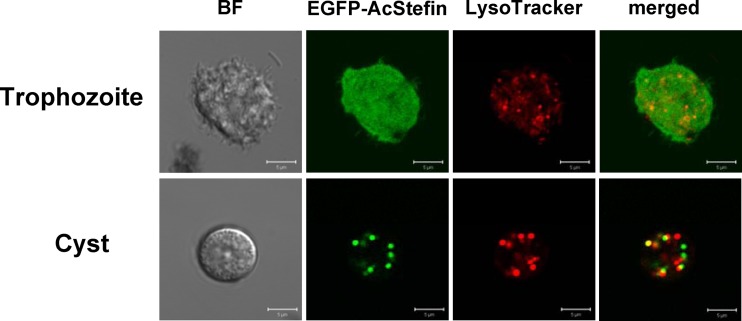
To determine the intracellular localization of AcStefin protein, Acanthamoeba trophozoites were transfected with an EGFP-fused AcStefin plasmid (pUb-EGFP-AcStefin); transferred into encystation medium; stained with LysoTracker Red; which labels acidic compartments such as lysosomes and autophagolysosomes; and examined by fluorescence microscopy (50, 51). As shown in Fig. 2, the distribution of AcStefin in trophozoites showed a dispersed pattern of fluorescence in the cytoplasm, indicating that EGFP-AcStefin is expressed in the cytoplasm of trophozoites. After transfected amoebae were transferred into encystment medium, the expressed EGFP-AcStefin fusion protein was also found to be partially colocalized with these LysoTracker-stained bodies, suggesting that some AcStefin is localized in Acanthamoeba cyst lysosomes or autophagolysosomes.
Fig 2.
Intracellular localization of AcStefin protein. Shown are subcellular localization changes in EGFP-fused AcStefin. Trophozoites were transfected with pUb-EGFP-AcStefin (green), transferred into a medium to induce encystation, incubated for 72 h, and examined under a fluorescence microscope. Lysosomes and autophagolysosomes were visualized by LysoTracker Red staining (red), and the resulting merged images are shown (yellow). BF denotes bright-field images. Bar = 5 μm.
Encystation and excystation of Acanthamoeba are not successful in AcStefin knockdown cells.
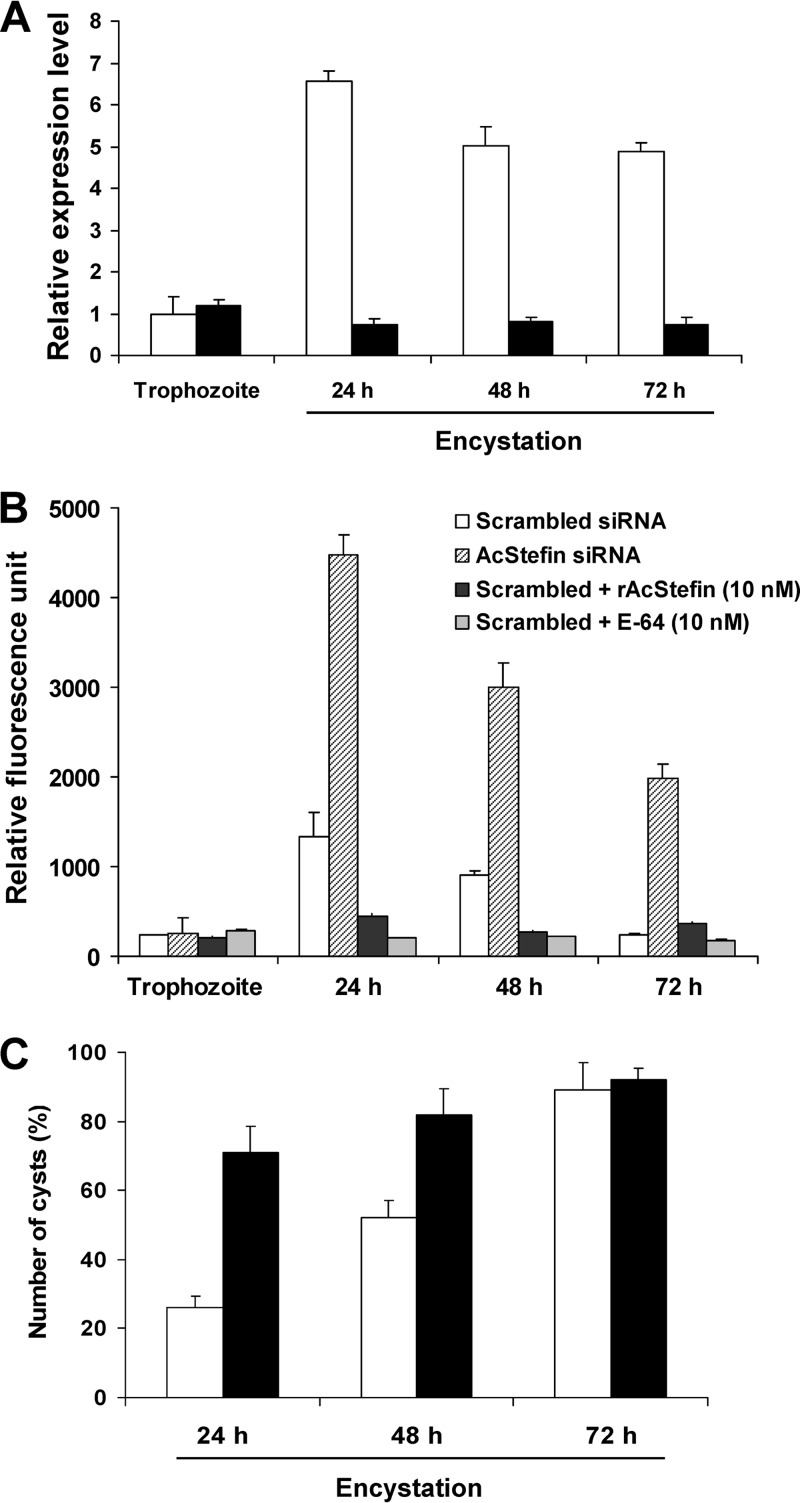
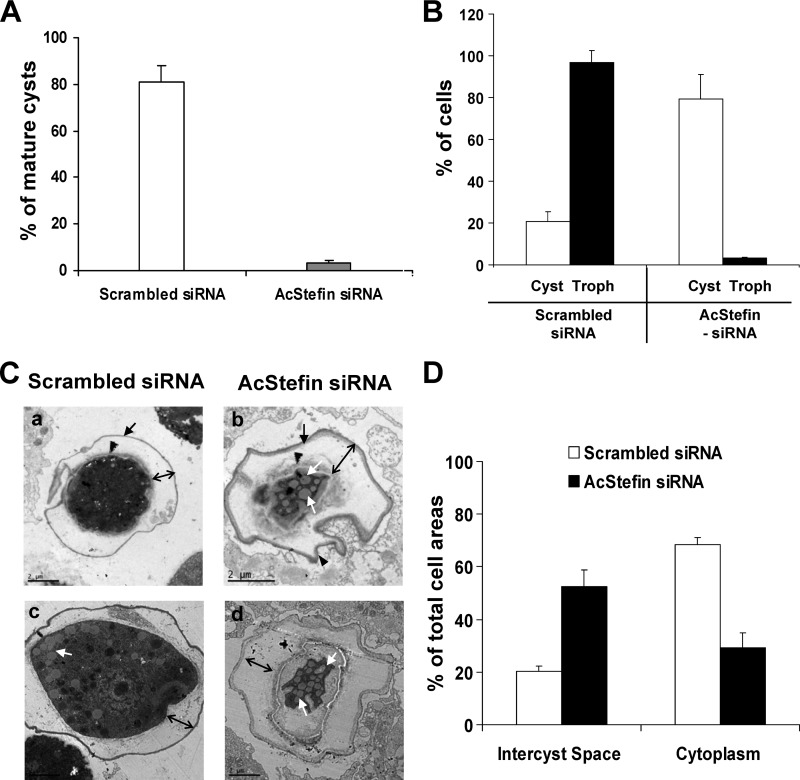
To determine the involvement of AcStefin in the encystation of Acanthamoeba, siRNA-mediated gene silencing was used to reduce the expression level of endogenous AcStefin. After transfection with siRNA against AcStefin or scrambled control siRNA, transfected trophozoites were transferred into encystation medium, and the transcriptional expression levels of AcStefin were examined by real-time PCR. As shown in Fig. 3A, AcStefin mRNA expression was markedly decreased in cysts at 24 h postencystation from AcStefin-siRNA-transfected trophozoites, and this was maintained for 72 h after encystation, indicating that siRNA-mediated gene knockdown was successful in these transfected cells. Next, we examined the effect of the siRNA-mediated silencing of AcStefin on cysteine protease activity in Acanthamoeba cysts by determining the specific activities of cathepsin L in scrambled-siRNA- and AcStefin-siRNA-transfected cells using fluorometric assays. As shown Fig. 3B, hydrolysis of cathepsin L increased at 24 h postencystation and gradually decreased at 48 h and 72 h in scrambled-siRNA-transfected cells, indicating that cathepsin L activity is increased during the early encystation stage. In AcStefin knockdown cells, cathepsin L activity was obvious increased versus that in scrambled-siRNA-transfected cells. Furthermore, these increased activities in cysts were reduced by adding recombinant AcStefin protein or E64, indicating that the observed increase in cathepsin L activity in AcStefin knockdown cells was indeed caused by AcStefin knockdown. To determine whether the enhancement of cysteine protease activity by AcStefin knockdown affects encystation, we counted cyst-like cells which showed the characteristic cyst wall under a microscope. Surprisingly, at 24 h after encystation induction, cysts comprised only 26% of the control population (scrambled-siRNA-transfected cell), whereas cysts comprised 76% of AcStefin knockdown cells (Fig. 3C). Next, we collected cyst-like cells by microscopy at 72 h postencystation. The same numbers of collected cysts from scrambled-siRNA- and AcStefin-siRNA-transfected cells were treated with Sarkosyl, given that mature cysts resist Sarkosyl exposure. After treatment, mature cysts comprised about 80% of the scrambled-siRNA-transfected cell populations, whereas very few mature cysts (<5%) were produced by AcStefin knockdown (Fig. 4A). The results indicate that despite a significant increase in cyst formation, most cyst-like cells observed among AcStefin knockdown cells by microscopy were immature or incomplete cysts. Moreover, the effects of AcStefin on subsequent excystation were also significant; that is, most scrambled-siRNA-transfected cells excysted, converted into viable trophozoites, and proliferated, whereas AcStefin-siRNA-transfected cells were almost arrested in the cystic stage even after 7 days of cultivation on E. coli-covered nonnutrient agar plates (Fig. 4B), indicating that incomplete cyst formation by AcStefin knockdown affects subsequent excystation. Thus, these results suggest that AcStefin upregulation is required to complete the encystation process via the modulation of cysteine proteases in Acanthamoeba cysts.
Fig 3.
Incomplete encystation by AcStefin siRNA. (A) Trophozoites were transfected with scrambled or AcStefin siRNA; transferred into encystment medium; incubated for 24 h, 48 h, or 72 h; and examined for transcriptional changes of AcStefin in scrambled siRNA (light bars) and AcStefin siRNA (dark bars) transfectants by real-time PCR. Transfection efficiencies (80 to 88%) of FITC-conjugated AcStefin siRNA were determined by fluorescence microscopy and standard fluorescence-activated cell sorting analysis. The expression of AcStefin was normalized to that of Acanthamoeba actin. (B) Cysteine protease activities of Acanthamoeba cysts. Cell extracts of scrambled-siRNA- and AcStefin-siRNA-transfected cells were prepared at 0, 24, 48, or 72 h postencystation. Cathepsin L activity was measured by using its specific substrate Z-Phe-Arg-AFC in the presence of recombinant AcStefin protein (10 nM) or E64 (10 nM). Data represent means ± standard deviations of experiments performed in triplicate. (C) Trophozoites transfected with scrambled siRNA (open bars) or with AcStefin siRNA (black bars) were incubated in encystment medium for 0, 24, 48, or 72 h, and numbers of mature cyst-like cells were counted under a microscope. The results show the means ± standard deviations of three separate experiments.
Fig 4.
Effects of AcStefin knockdown on encystation and excystation. (A) The same numbers of mature cyst-like cells with a cyst wall of scrambled siRNA transfectants (control) (light bar) and AcStefin-siRNA transfectants (dark bar) were collected under a microscope at 72 h postencystation and then treated with 0.05% Sarkosyl for 30 min. Sarkosyl-resistant mature cysts were stained with 0.22% trypan blue and counted under a microscope. (B) The same numbers of mature cyst-like cells were transferred onto E. coli-covered nonnutrient agar plates and incubated for 7 days at 27°C, and trophozoites (light bars) and cysts (dark bars) were counted for control (scrambled-siRNA-transfected) and AcStefin-siRNA-transfected (AcStefin-siRNA) cells. Value represent the means ± standard deviations of triplicate experiments. (C) Representative TEM images showing a thick intercyst space and reductions in the cytoplasmic areas of AcStefin knockdown cells. Cells transfected with nonspecific scrambled siRNA (a and c) and AcStefin siRNA (b and d) were transferred into encystment medium, incubated for 72 h, and examined by TEM. The black arrows and arrowheads show the exocyst wall and the endocyst wall in both scrambled-siRNA- and AcStefin-siRNA-transfected cells, respectively. The black double-headed arrows indicate the intercyst space. The white arrows indicate lipid droplets. Bar = 2 μm. (D) Intercyst space and cytoplasmic areas were measured. Pixel areas were determined by using ImageJ analysis tools, using 30 cysts of scrambled-siRNA- or AcStefin-siRNA-transfected cells in each of 10 TEM pictures.
Ultrastructural changes in AcStefin knockdown cells.
Ultrastructural changes of AcStefin knockdown cells were examined by TEM (Fig. 4C). The mature cyst wall of scrambled-siRNA-transfected cells was constituted of two major layers, a laminar fibrous exocyst (Fig. 4Ca and b, black arrows) and an endocyst composed of fine fibrils in a granular matrix (Fig. 4Ca and b, black arrowheads), which are typically detected in Acanthamoeba cysts (52). In AcStefin-siRNA-transfected cells, exocyst and endocyst are normally found in mature cysts, but the exocyst wall was thicker than and not as compact as that of scrambled-siRNA-transfected cells. In AcStefin-siRNA-transfected cells, the intercyst space separating these two layers (52) was noticeably larger than that of scrambled-siRNA-transfected cells (Fig. 4C, double-headed arrows). Although total cell areas of AcStefin-siRNA-transfected cells were not significantly changed compared with those of scrambled-siRNA-transfected cells, the calculated area of intercyst space revealed ∼30% increases in AcStefin-siRNA-transfected cells (Fig. 4D). In contrast to intercyst spaces, cytoplasmic areas in AcStefin-siRNA-transfected cells were significantly lower (by 40%) than those in scrambled-siRNA-transfected cells (Fig. 4Cb and d and D), indicating that the markedly greater intercyst space in AcStefin knockdown cells was caused by a reduction in the cytoplasmic area. Furthermore, cytosol degradation in AcStefin knockdown cells was severe, and only some lipid droplets remained (43) (Fig. 4Cc and d, white arrows). These results suggest that ultrastructural changes in AcStefin knockdown cells were caused by cytosolic degradation by cysteine proteases. Collectively, our findings support the view that AcStefin is essential for encystation and that AcStefin regulates intracellular cysteine protease activity during encystation.
DISCUSSION
Cysteine proteases in lysosomes are involved in many cellular processes and are key players in the macromolecular turnover associated with the degradation of the cellular components (7, 53). Recent studies of Giardia and Entamoeba have revealed that cysteine proteases are implicated in encystation (9, 11, 54–56). In Giardia, the expressions of encystation-specific cysteine proteases (ESCPs) (a cathepsin C family of enzymes) are developmentally regulated during encystation (57). Entamoeba invadens EiCP-B9, a cyst-specific cysteine protease, is expressed near the cyst wall of immature cysts and throughout mature cysts (11). The inhibition of the activities of cysteine proteases during encystation by cysteine protease inhibitors such as E64 or Z-Phe-Arg-CH2F reduces the cyst-forming ability of E. invadens (13, 14). In the present study, the level of cathepsin L activity of cyst lysates was elevated at 24 h postencystation and was inhibited by treatment with recombinant AcStefin or E64, indicating that the activities of cysteine proteases are indeed highly upregulated during the encystation of Acanthamoeba.
In the present study, we found that AcStefin is a cysteine protease inhibitor in A. castellanii, and we examined its inhibitory effect on cysteine proteases and on the encystation of Acanthamoeba. Because AcStefin is highly expressed in cysts, we upregulated the activities of cysteine proteases by AcStefin knockdown and examined the resulting effects on Acanthamoeba encystation and excystation. Cysteine protease activities were found to be greater in AcStefin knockdown cysts than in control Acanthamoeba cysts. Interestingly, encystment appeared to be accelerated by AcStefin knockdown, but most AcStefin knockdown cells exhibited incomplete cyst formation, as only a few mature cysts were present, as determined by Sarkosyl treatment. Furthermore, TEM-based ultrastructural analysis revealed defects in exocyst walls and significant cytoplasmic degradation, which favors the view that in Acanthamoeba, cysteine protease regulation is required for successful cyst formation by preventing the proteolytic degradation of intracellular components required for subsequent excystation.
Several cystatins have been found to inhibit cysteine proteases and to have immunomodulatory effects on nematodes (20–27), and the regulatory effects of intracellular cysteine proteases have been found to protect against proteolytic damage in trematodes (28, 29). In protozoan parasites, endogenous protease inhibitors have been found to regulate protease activity by blocking deleterious effects of host proteases from Plasmodium falciparum (34), Trypanosoma cruzi (31), Leishmania mexicana (32), and Entamoeba (36). In Plasmodium, falstatin, a cysteine protease inhibitor of P. falciparum, is required to facilitate red cell invasion (34), and PbICP (Plasmodium berghei inhibitor of cysteine proteases) has been reported to play important roles in sporozoite invasion and host cell survival (35). In T. cruzi, the overexpression of chagasin, an inhibitor of cysteine proteases (including cruzain), decreased infectivity in cell culture (31), and in L. mexicana, virulence in mice was markedly abolished by disrupting the activities of cysteine protease inhibitors (32). However, these cysteine protease inhibitors were classified as members of the chagasins (family I42), and thus, no member of the cystatins (family I25) has yet been identified in protozoan parasites. Accordingly, to the best of our knowledge, this is the first report of a cystatin of family I25 in a protozoan parasite.
In our previous studies, we determined that autophagy is essential for the encystation of Acanthamoeba (58, 59), and it is likely that the higher expression levels of cysteine proteases during encystation are associated with degradation of intracellular contents in autophagosomes (60). Murine cystatin C (CysC) is known to play a protective role under conditions of neuronal challenge by inducing autophagy via the mTOR pathway, which leads to enhanced proteolytic clearance of autophagy substrates by lysosomes, whereas the inhibitory effects of CysC on cathepsin B are not required for these neuroprotective functions (61). Although in the present study, suppression of AcStefin expression caused incomplete cyst formation and significantly diminished excystation efficiency in Acanthamoeba, we did not determine whether AcStefin induces autophagy via a specific signaling pathway in Acanthamoeba. Recently, we identified a cysteine protease that is significantly upregulated during encystation and found it to be associated with lysosomes and autophagolysosomes (62). However, the identities of the cysteine protease targets of AcStefin were not determined in the present study. Also, our findings do not exclude the possibility that the cysteine proteases of trophozoites, and not those of cysts, are inhibited by AcStefin during encystation. Thus, further studies are required to address these questions and to determine the mechanism responsible for Acanthamoeba encystation at the molecular level.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0068573) and Kyungpook National University Research Fund, 2012.
Footnotes
Published ahead of print 8 February 2013
REFERENCES
- 1. Band RN, Mohrlok S. 1973. The cell cycle and induced amitosis in Acanthamoeba. J. Protozool. 20:654–657 [DOI] [PubMed] [Google Scholar]
- 2. Neff RJ, Neff RH. 1969. The biochemistry of amoebic encystment. Symp. Soc. Exp. Biol. 23:51–81 [PubMed] [Google Scholar]
- 3. Lloyd D, Turner NA, Khunkitti W, Hann AC, Furr JR, Russell AD. 2001. Encystation in Acanthamoeba castellanii: development of biocide resistance. J. Eukaryot. Microbiol. 48:11–16 [DOI] [PubMed] [Google Scholar]
- 4. Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan NA. 2009. Acanthamoeba: biology and pathogenesis. Caister Academic, Norfolk, United Kingdom [Google Scholar]
- 6. McClellan K, Howard K, Mayhew E, Niederkorn J, Alizadeh H. 2002. Adaptive immune responses to Acanthamoeba cysts. Exp. Eye Res. 75:285–293 [PubMed] [Google Scholar]
- 7. McGrath ME. 1999. The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct. 28:181–204 [DOI] [PubMed] [Google Scholar]
- 8. Sajid M, McKerrow JH. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1–21 [DOI] [PubMed] [Google Scholar]
- 9. DuBois KN, Abodeely M, Sakanari J, Craik CS, Lee M, McKerrow JH, Sajid M. 2008. Identification of the major cysteine protease of Giardia and its role in encystation. J. Biol. Chem. 283:18024–18031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ward W, Alvarado L, Rawlings ND, Engel JC, Franklin C, McKerrow JH. 1997. A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell 89:437–444 [DOI] [PubMed] [Google Scholar]
- 11. Ebert F, Bachmann A, Nakada-Tsukui K, Hennings I, Drescher B, Nozaki T, Tannich E, Bruchhaus I. 2008. An Entamoeba cysteine peptidase specifically expressed during encystation. Parasitol. Int. 57:521–524 [DOI] [PubMed] [Google Scholar]
- 12. Van Dellen KL, Chatterjee A, Ratner DM, Magnelli PE, Cipollo JF, Steffen M, Robbins PW, Samuelson J. 2006. Unique posttranslational modifications of chitin-binding lectins of Entamoeba invadens cyst walls. Eukaryot. Cell 5:836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez J, Bai G, Frevert U, Corey EJ, Eichinger D. 1999. Proteasome-dependent cyst formation and stage-specific ubiquitin mRNA accumulation in Entamoeba invadens. Eur. J. Biochem. 264:897–904 [DOI] [PubMed] [Google Scholar]
- 14. Sharma M, Hirata K, Herdman S, Reed S. 1996. Entamoeba invadens: characterization of cysteine proteinases. Exp. Parasitol. 84:84–91 [DOI] [PubMed] [Google Scholar]
- 15. Leitsch D, Kohsler M, Marchetti-Deschmann M, Deutsch A, Allmaier G, Duchene M, Walochnik J. 2010. Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment. Eukaryot. Cell 9:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moon EK, Chung DI, Hong YC, Ahn TI, Kong HH. 2008. Acanthamoeba castellanii: gene profile of encystation by ESTs analysis and KOG assignment. Exp. Parasitol. 119:111–116 [DOI] [PubMed] [Google Scholar]
- 17. Moon EK, Xuan YH, Chung DI, Hong Y, Kong HH. 2011. Microarray analysis of differentially expressed genes between cysts and trophozoites of Acanthamoeba castellanii. Korean J. Parasitol. 49:341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turk V, Bode W. 1991. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 285:213–219 [DOI] [PubMed] [Google Scholar]
- 19. Rawlings ND, Tolle DP, Barrett AJ. 2004. MEROPS: the peptidase database. Nucleic Acids Res. 32:D160–D164 doi:10.1093/nar/gkh071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hartmann S, Kyewski B, Sonnenburg B, Lucius R. 1997. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur. J. Immunol. 27:2253–2260 [DOI] [PubMed] [Google Scholar]
- 21. Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, Takashima M, Himeno K. 2001. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect. Immun. 69:7380–7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manoury B, Gregory WF, Maizels RM, Watts C. 2001. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol. 11:447–451 [DOI] [PubMed] [Google Scholar]
- 23. Schonemeyer A, Lucius R, Sonnenburg B, Brattig N, Sabat R, Schilling K, Bradley J, Hartmann S. 2001. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J. Immunol. 167:3207–3215 [DOI] [PubMed] [Google Scholar]
- 24. Pfaff AW, Schulz-Key H, Soboslay PT, Taylor DW, MacLennan K, Hoffmann WH. 2002 Litomosoides sigmodontis cystatin acts as an immunomodulator during experimental filariasis. Int. J. Parasitol. 32:171–178 [DOI] [PubMed] [Google Scholar]
- 25. Hartmann S, Schonemeyer A, Sonnenburg B, Vray B, Lucius R. 2002. Cystatins of filarial nematodes up-regulate the nitric oxide production of interferon-gamma-activated murine macrophages. Parasite Immunol. 24:253–262 [DOI] [PubMed] [Google Scholar]
- 26. Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. 2003. Parasite-specific immunomodulatory functions of filarial cystatin. Infect. Immun. 71:2422–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray J, Manoury B, Balic A, Watts C, Maizels RM. 2005. Bm-CPI-2, a cystatin from Brugia malayi nematode parasites, differs from Caenorhabditis elegans cystatins in a specific site mediating inhibition of the antigen-processing enzyme AEP. Mol. Biochem. Parasitol. 139:197–203 [DOI] [PubMed] [Google Scholar]
- 28. Tarasuk M, Vichasri Grams S, Viyanant V, Grams R. 2009. Type I cystatin (stefin) is a major component of Fasciola gigantica excretion/secretion product. Mol. Biochem. Parasitol. 167:60–71 [DOI] [PubMed] [Google Scholar]
- 29. Kang JM, Lee KH, Sohn WM, Na BK. 2011. Identification and functional characterization of CsStefin-1, a cysteine protease inhibitor of Clonorchis sinensis. Mol. Biochem. Parasitol. 177:126–134 [DOI] [PubMed] [Google Scholar]
- 30. Vray B, Hartmann S, Hoebeke J. 2002. Immunomodulatory properties of cystatins. Cell. Mol. Life Sci. 59:1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monteiro AC, Abrahamson M, Lima AP, Vannier-Santos MA, Scharfstein J. 2001. Identification, characterization and localization of chagasin, a tight-binding cysteine protease inhibitor in Trypanosoma cruzi. J. Cell Sci. 114:3933–3942 [DOI] [PubMed] [Google Scholar]
- 32. Besteiro S, Coombs GH, Mottram JC. 2004. A potential role for ICP, a leishmanial inhibitor of cysteine peptidases, in the interaction between host and parasite. Mol. Microbiol. 54:1224–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang R, Que X, Hirata K, Brinen LS, Lee JH, Hansell E, Engel J, Sajid M, Reed S. 2009. The cathepsin L of Toxoplasma gondii (TgCPL) and its endogenous macromolecular inhibitor, toxostatin. Mol. Biochem. Parasitol. 164:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey KC, Singh N, Arastu-Kapur S, Bogyo M, Rosenthal PJ. 2006. Falstatin, a cysteine protease inhibitor of Plasmodium falciparum, facilitates erythrocyte invasion. PLoS Pathog. 2:e117 doi:10.1371/journal.ppat.0020117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rennenberg A, Lehmann C, Heitmann A, Witt T, Hansen G, Nagarajan K, Deschermeier C, Turk V, Hilgenfeld R, Heussler VT. 2010. Exoerythrocytic Plasmodium parasites secrete a cysteine protease inhibitor involved in sporozoite invasion and capable of blocking cell death of host hepatocytes. PLoS Pathog. 6:e1000825 doi:10.1371/journal.ppat.1000825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riekenberg S, Witjes B, Saric M, Bruchhaus I, Scholze H. 2005. Identification of EhICP1, a chagasin-like cysteine protease inhibitor of Entamoeba histolytica. FEBS Lett. 579:1573–1578 [DOI] [PubMed] [Google Scholar]
- 37. Rigden DJ, Mosolov VV, Galperin MY. 2002. Sequence conservation in the chagasin family suggests a common trend in cysteine proteinase binding by unrelated protein inhibitors. Protein Sci. 11:1971–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanderson SJ, Westrop GD, Scharfstein J, Mottram JC, Coombs GH. 2003. Functional conservation of a natural cysteine peptidase inhibitor in protozoan and bacterial pathogens. FEBS Lett. 542:12–16 [DOI] [PubMed] [Google Scholar]
- 39. Santos CC, Scharfstein J, Lima PCA. 2006. Role of chagasin-like inhibitors as endogenous regulators of cysteine proteases in parasitic protozoa. Parasitol. Res. 99:323–324 [DOI] [PubMed] [Google Scholar]
- 40. Santos CC, Sant'Anna C, Terres A, Cunha-e-Silva NL, Scharfstein J, Lima PCA. 2005. Chagasin, the endogenous cysteine-protease inhibitor of Trypanosoma cruzi, modulates parasite differentiation and invasion of mammalian cells. J. Cell Sci. 118:901–915 [DOI] [PubMed] [Google Scholar]
- 41. Saric M, Vahrmann A, Bruchhaus I, Bakker-Grunwald T, Scholze H. 2006. The second cysteine protease inhibitor, EhICP2, has a different localization in trophozoites of Entamoeba histolytica than EhICP1. Parasitol. Res. 100:171–174 [DOI] [PubMed] [Google Scholar]
- 42. Sato D, Nakada-Tsukui K, Okada M, Nozaki T. 2006. Two cysteine protease inhibitors, EhICP1 and 2, localized in distinct compartments, negatively regulate secretion in Entamoeba histolytica. FEBS Lett. 580:5306–5312 [DOI] [PubMed] [Google Scholar]
- 43. Bowers B, Korn ED. 1969. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J. Cell Biol. 41:786–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Picazarri K, Nakada-Tsukui K, Nozaki T. 2008. Autophagy during proliferation and encystation in the protozoan parasite Entamoeba invadens. Infect. Immun. 76:278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazur T, Zozwiak M. 1989. Value of the color test in assessing the viability of cysts of Acanthamoeba sp. Wiad. Parazytol. 35:11–17 [PubMed] [Google Scholar]
- 46. Nellen W, Gallwitz D. 1982. Actin genes and actin messenger RNA in Acanthamoeba castellanii. Nucleotide sequence of the split actin gene I. J. Mol. Biol. 159:1–18 [DOI] [PubMed] [Google Scholar]
- 47. Kong HH, Pollard TD. 2002. Intracellular localization and dynamics of myosin-II and myosin-IC in live Acanthamoeba by transient transfection of EGFP fusion proteins. J. Cell Sci. 115:4993–5002 [DOI] [PubMed] [Google Scholar]
- 48. Hsieh WT, Fong D, Sloane BF, Golembieski W, Smith DI. 1991. Mapping of the gene for human cysteine proteinase inhibitor stefin A, STF1, to chromosome 3cen-q21. Genomics 9:207–209 [DOI] [PubMed] [Google Scholar]
- 49. Stubbs MT, Laber B, Bode W, Huber R, Jerala R, Lenarcic B, Turk V. 1990. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 9:1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scott RC, Schuldiner O, Neufeld TP. 2004. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7:167–178 [DOI] [PubMed] [Google Scholar]
- 51. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, et al. 2008. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4:151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weisman RA. 1976. Differentiation in Acanthamoeba castellanii. Annu. Rev. Microbiol. 30:189–219 [DOI] [PubMed] [Google Scholar]
- 53. Bruchhaus I, Loftus BJ, Hall N, Tannich E. 2003. The intestinal protozoan parasite Entamoeba histolytica contains 20 cysteine protease genes, of which only a small subset is expressed during in vitro cultivation. Eukaryot. Cell 2:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lujan HD, Mowatt MR, Nash TE. 1997. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol. Mol. Biol. Rev. 61:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hehl AB, Marti M, Kohler P. 2000. Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol. Biol. Cell 11:1789–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reiner DS, McCaffery M, Gillin FD. 1990. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur. J. Cell Biol. 53:142–153 [PubMed] [Google Scholar]
- 57. Touz MC, Nores MJ, Slavin I, Carmona C, Conrad JT, Mowatt MR, Nash TE, Coronel CE, Lujan HD. 2002. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 277:8474–8481 [DOI] [PubMed] [Google Scholar]
- 58. Moon EK, Chung DI, Hong YC, Kong HH. 2009. Autophagy protein 8 mediating autophagosome in encysting Acanthamoeba. Mol. Biochem. Parasitol. 168:43–48 [DOI] [PubMed] [Google Scholar]
- 59. Song SM, Han BI, Moon EK, Lee YR, Yu HS, Jha BK, Danne DB, Kong HH, Chung DI, Hong Y. 2012. Autophagy protein 16-mediated autophagy is required for the encystation of Acanthamoeba castellanii. Mol. Biochem. Parasitol. 183:158–165 [DOI] [PubMed] [Google Scholar]
- 60. Band RN, Mohrlok S. 1973. Observations on induced amitosis in Acanthamoeba. Exp. Cell Res. 79:327–337 [DOI] [PubMed] [Google Scholar]
- 61. Tizon B, Sahoo S, Yu H, Gauthier S, Kumar AR, Mohan P, Figliola M, Pawlik M, Grubb A, Uchiyama Y, Bandyopadhyay U, Cuervo AM, Nixon RA, Levy E. 2010. Induction of autophagy by cystatin C: a mechanism that protects murine primary cortical neurons and neuronal cell lines. PLoS One 5:e9819 doi:10.1371/journal.pone.0009819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moon EK, Hong Y, Chung DI, Kong HH. 2012. Cysteine protease involving in autophagosomal degradation of mitochondria during encystation of Acanthamoeba. Mol. Biochem. Parasitol. 185:121–126 [DOI] [PubMed] [Google Scholar]