Abstract
An expert group of 40 pain specialists from 16 countries performed a first assessment of the value of predictors for treatment success with 5% lidocaine-medicated plaster in the management of cancer pain with neuropathic components and trigeminal neuropathic pain. Results were based on the retrospective analysis of 68 case reports (sent in by participants in the 4 weeks prior to the conference) and the practical experience of the experts. Lidocaine plaster treatment was mostly successful for surgery or chemotherapy-related cancer pain with neuropathic components. A dose reduction of systemic pain treatment was observed in at least 50% of all cancer pain patients using the plaster as adjunct treatment; the presence of allodynia, hyperalgesia or pain quality provided a potential but not definitively clear indication of treatment success. In trigeminal neuropathic pain, continuous pain, severe allodynia, hyperalgesia, or postherpetic neuralgia or trauma as the cause of orofacial neuropathic pain were perceived as potential predictors of treatment success with lidocaine plaster. In conclusion, these findings provide a first assessment of the likelihood of treatment benefits with 5% lidocaine-medicated plaster in the management of cancer pain with neuropathic components and trigeminal neuropathic pain and support conducting large, well-designed multicenter studies.
Keywords: lidocaine plaster, neuropathic pain, cancer pain, trigeminal neuropathic pain, case reports
Background
Neuropathic pain that “arises as a direct consequence of a lesion or disease affecting the somatosensory system”1,2 is experienced by 6%–8% of adults in the general population.3 Prevalence can vary according to the underlying condition, eg, approximately 20% of patients with long-standing diabetes, and approximately 8% of individuals who suffered from shingles are affected.3 The risk is higher in older adults, because the incidence of many diseases causing neuropathic pain increases with age.4 Pharmacotherapy remains the most important treatment option5 but three-quarters of patients in cross-sectional surveys still had moderate to severe pain despite taking medications.6
The topical analgesic 5% lidocaine-medicated plaster (Versatis®; Grünenthal, Aachen, Germany) is recommended as first-line treatment for localized peripheral neuropathic pain.7,8 The lidocaine plaster has shown good efficacy and tolerability in patients with postherpetic neuralgia,9–11 diabetic polyneuropathy,9 and other neuropathic pain states.12–15 Pain relief was observed for up to 7 treatment years with daily plaster use.16–20 Recently, a decrease in the painful surface area following lidocaine plaster treatment was demonstrated for the first time in a prospective clinical study21 and confirmed in surrogate neuropathic pain models in healthy volunteers.22
Currently, attempts are underway to supplement the traditional classification of neuropathic pain (based on disease entities, anatomical localization or histological observations) by a mechanism- or symptom-based classification.23 The efficacy of the lidocaine plaster in different neuropathic pain conditions has led to the hypothesis of a common localized symptomatology that might provide common predictors of treatment success. The availability of positive- and negative-outcome predictors for a certain treatment might shorten the “trial and error” period in finding a successful treatment for a patient, thus providing pain relief and better quality of life faster. Previous meetings of pain specialists in 2007, 2008, and 2009 focused on potential outcome predictors for the indications diabetic polyneuropathy (DPN), complex regional pain syndrome (CRPS), chronic low back pain with neuropathic components, and chronic neuropathic pain after surgical and nonsurgical trauma. Based on case reports and clinical experience of the participants, presence of localized pain, allodynia, hyperalgesia, and superficial pain were considered positive predictors for treatment success with the lidocaine plaster, whereas the predictive value of pain quality differed depending on the indication and was considered uncertain. Treatment success was generally considered unlikely in the presence of chronic widespread pain, deep pain, or numbness.24 The probability of treatment success with the lidocaine plaster for cancer pain with neuropathic components and trigeminal neuropathic pain has so far been considered mainly on anecdotal evidence. Pain specialists experienced in lidocaine plaster treatment of one or both pain states therefore discussed possible outcome predictors for these two indications at an additional 2-day meeting held in 2010.
Cancer pain with neuropathic components
Pain is prevalent in cancer patients and considerably impairs their quality of life.25 The pain is often experienced at multiple sites and tends to increase in severity with advancing disease. Cancer pain can be nociceptive or neuropathic; patients often present with a mixed nociceptive/neuropathic type.26,27 The prevalence of neuropathic pain in cancer pain has been estimated at between 11.8% and 33%,28–30 but could be higher, as patients with mixed pain were not included in the neuropathic pain estimate in one study.29 Neuropathic pain can arise from nerve compression or direct tumor infiltration or can be induced by cancer treatments such as surgery, radiotherapy, or chemotherapy.31,32 In some cases, the pain is cancer-independent and caused by concomitant disorders. Pharmacological management of cancer pain usually follows the World Health Organization’s analgesic ladder for cancer pain relief;33 however, this approach does not take into account the special aspects of neuropathic pain. Cancer pain is difficult to manage, especially in patients with neuropathic pain components;34 the use of adjuvant analgesics with proven efficacy in the management of neuropathic pain8 is therefore recommended. So far, lidocaine plaster treatment of cancer patients with neuropathic pain has provided analgesic efficacy in some cases35,36 but not in others.37
Trigeminal neuropathic pain
Facial pain is a rare but severe condition with an incidence rate of 38.7 per 100,000 person years;38 it has a profound effect on quality of life.39 Trigeminal neuralgia is the most widely recognized neuropathic facial pain, but there are other neuropathic pain types that require a different treatment regimen.40 Criteria of the International Association for the Study of Pain2 or of the International Classification of Headache Disorders41 are used for diagnosis and classification, which can be challenging because signs and symptoms of different pain types can overlap considerably.42,43 Misdiagnosis by general practitioners (nonspecialists in this field) can thus be substantial.38 One neuropathic pain type is trigeminal neuropathic pain, which needs to be differentiated from trigeminal neuralgia and requires a different treatment approach. A comparison of diagnostic criteria for the two pain types is listed in a recent publication.39 Trigeminal neuropathic pain is described as “aching throbbing” or burning pain around a tooth or area of past dental trauma/surgery or facial trauma, and is continuous soon after the injury occurred (in contrast to trigeminal neuralgia pain, which is described as sudden, brief, and extremely painful pain attacks).39 However, some forms of trigeminal neuralgia have a more prolonged afterpain, which has been termed either atypical trigeminal neuralgia or more recently type 2 trigeminal neuralgia.44 Trigeminal neuropathic pain can be localized or may radiate, is evoked by light touch, and associated with allodynia. Also described in the literature is a chronic dental pain called atypical odontalgia,45 which may constitute a subtype of trigeminal neuropathic pain.40 For pharmacological treatment, it is recommended to follow the guidelines used for the management of neuropathic pain,8 which include tricyclic antidepressants, calcium channel blockers (gabapentin and pregabalin), the serotonin–norepinephrine reuptake inhibitors duloxetine and venlafaxine, and the 5% lidocaine-medicated plaster.40 To our knowledge, use of the lidocaine plaster has so far only been described in a retrospective analysis of the management of iatrogenic trigeminal nerve injury.46
Meeting details
During a 2-day meeting facilitated by Grünenthal in December 2010 in Aachen, Germany, pain specialists experienced in the use of the 5% lidocaine-medicated plaster treatment for the management of cancer pain with neuropathic components and/or trigeminal neuropathic pain discussed possible predictors for a treatment response to the lidocaine plaster. The discussions were based on the retrospective analysis of case reports and the practical experience of the participants. Cases were discussed, and if there was a lack of agreement on the quality of data, they were excluded. Forty pain practitioners from 16 countries participated. They formed two discussion groups for lidocaine plaster use in cancer pain with neuropathic components, and one group for use in trigeminal neuropathic pain, each moderated by the authors of this paper.
Approximately 4 weeks prior to the meeting, all participants were asked to contribute case reports for one or both of the clinical indications using standardized and anonymized forms. This time frame allowed retrospective data analysis, but was insufficient for starting treatment in new patients for the purpose of data collection, which was not permitted. During the meeting, it became evident that additional information pertaining to the cause of neuropathic pain was considered useful for the analysis. Additional questions referring to the initially supplied case reports were therefore sent out to all contributing practitioners shortly after the meeting. Overall, the following data were obtained:
Demographic data.
Primary diagnosis (pain indication/cause of pain).
Other relevant diagnoses.
Cause of neuropathic pain; was pain related to the underlying condition or was it treatment-induced?
Localization of pain symptoms.
Duration and intensity of pain prior to initiation of treatment with lidocaine plaster.
Physical examination and diagnostic tests.
Presence of clinical symptoms of pain and pain quality (hyperalgesia, presence and severity of allodynia, stabbing pain, burning pain, shooting pain, other symptoms).
Prior and concomitant medication.
Start of therapy with lidocaine plaster.
Application frequency, number of plasters, and duration of treatment.
Clinical Global Impression of Change (CGIC) score during treatment with lidocaine plaster (from 1 = very much improved to 6 = very much worse).
occurrence of adverse events.
conclusions of the practitioner.
The presence of hyperalgesia (increased pain sensitivity in response to nociceptive stimuli) and allodynia (pain response to nonnociceptive stimuli) was confirmed by the treating physician using diagnostic tools of his/her choice. Allodynia severity was rated on a scale from 0 = no pain or discomfort to touch, 1 = uncomfortable, but tolerable to touch, 2 = painful, to 3 = extremely painful, patient cannot stand touching.
Sixty-eight case reports were submitted by 18 pain practitioners from the following countries: Belgium (2), Czech Republic (1), France (3), Germany (2), Poland (2), Portugal (3), Slovakia (1), Slovenia (1), Spain (1), The Netherlands (1), and the United Kingdom (1). All reports were displayed during the discussion sessions and tabulated according to indication. The original report forms were also available for perusal at each session. Group participants jointly reviewed and discussed these reports, and progressed from this basis to an exchange of experience regarding outcome predictors. Final conclusions were drawn collectively following group discussions. Three cases with insufficient data were not included in this analysis.
Cancer pain with neuropathic components
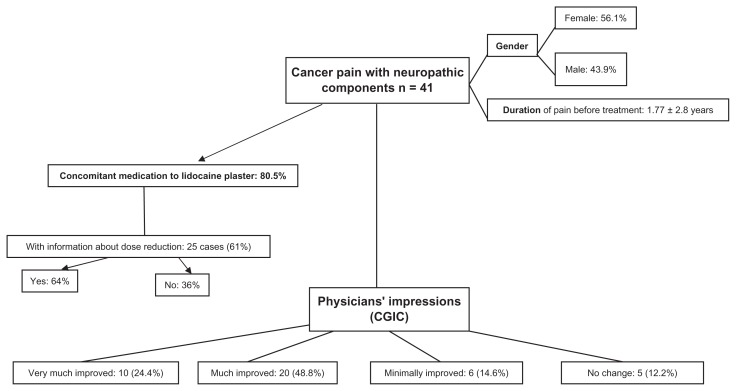
Forty-one cases of cancer pain with neuropathic components were reviewed (Figure 1). The mean age of the patients was 59.5 ± 13.7 years, with a slightly higher proportion of females (56.1%). Mean duration of pain before treatment with the lidocaine plaster was 1.77 ± 2.8 years. The majority of patients (80.5%) received concomitant pain medication during lidocaine plaster treatment. Dose reductions of concomitant medication were reported for 64% of the 25 cases with available information for this parameter. Physicians judged 20 patients (48.8%) as much improved (CGIC = 2) and ten patients (24.4%) as very much improved (CGIC = 1) following lidocaine plaster treatment. Six patients showed minimal improvement and no change was reported for five patients.
Figure 1.
Forty-one case reports of patients with cancer pain with neuropathic components were reviewed.
Abbreviation: CGIC, Clinical Global Impression of Change.
Hyperalgesia and allodynia were present in 53.7% and 70.7% of all cases, respectively. Burning pain was reported for 26 patients (63.4%) and shooting pain for 21 patients (51.2%). The majority of the hyperalgesia patients (72.7%) showed at least much improvement following plaster treatment, but so also did most of the patients without hyperalgesia (73.7%). A slight trend towards improved outcome was observed for patients with allodynia (75.9% vs 66.7% for patients without allodynia) and for patients with burning pain (76.9% vs 66.7% for patients without burning pain). A slight negative trend was observed for patients with shooting pain (66.7% vs 80%). Nineteen patients suffered from severe allodynia (painful or extremely painful); 15 of those (78.9%) were at least much improved.
From these results, neither hyperalgesia, allodynia, burning pain, nor shooting pain can be regarded as a clear outcome predictor for lidocaine plaster in the treatment of cancer pain with neuropathic components. However, the participants in one discussion group concluded from their clinical experience that hyperalgesia and allodynia (except cold allodynia) are potential positive predictors of treatment success with lidocaine plaster. When deciding on possible plaster treatment, they would use these as positive predictors and widespread pain, anesthesia/numbness, and cold allodynia as negative predictors. In contrast, the other discussion group regarded allodynia and burning pain as potentially helpful but not sufficient, and had varying experiences concerning anesthesia. In their view, the ability of the patient to describe and localize the pain area may serve as a positive predictor.
In general, this group thought that the case reports collected prior to the meeting did not contain enough information about the cause of the neuropathic pain to cluster cases and to draw definite conclusions. Information regarding a relationship of the experienced neuropathic pain to treatment (chemotherapy, radiotherapy, surgery) or to the cancer (ie, compression, infiltration) was thus sought after the meeting. Physicians kindly provided details, and all cases were stratified by relationship to treatment or cancer (Tables 1–4).
Table 1.
Case reports for cancer pain with neuropathic components related to chemotherapy (pain was additionally related to radiotherapy in one patient and to surgery and cancer in another)
| Gender /case number | Age | Primary diagnosis (pain indication/cause of pain) | Localization of pain symptoms | Duration of pain in years | Hyper-algesia | Allodynia/ allodynia severity rating | Pain intensity (0–10) | Pain quality | Mono-therapy | Plasters per day | Duration of plaster treatment (months) | CGIC score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M C41 |
57 | Prostate cancer, bone metastases; osteonecrosis of jaw due to chemotherapy | Left chin | <1 | 5 | Stabbing, shooting, like electric discharges very short but intense | N | 1/8 | 1 month, ongoing | 1 | ||
| M C5 |
60 | Lung adenocarcinoma | Mainly feet, thoracotomy | 1.5 | X | 1 | 4–5 | Burning | N | 3 | 2 | 2 |
| Fa C6 |
48 | Ovarian cancer (post chemotherapy and surgery), other relevant diagnosis: CRPS 1, 2 months after chemotherapy | Hands, mainly right | 0.25 | X | 2 | 7 | Stabbing | N | 2 | 0.5 | 2 |
| Fa C7 |
43 | CRPS 1 (legs) 3 years post nephrectomy, renal implantation, chemotherapy | Feet | 0.83 | X | 3 | 8–9 | Shooting | Y | 3 | 0.67 | 2 |
| Mb C8 |
68 | Planoepithelial carcinoma right lung (complete resection and chemo) | Thoracic region (scar) | 0.5 | 1 | 6 | Shooting | Y | 1 | 0.5 | 2 | |
| F C18 |
60 | Chemotherapy oxaliplatin | Hands and feet | 1.67 | 7–8 | Tingling | Y | 4 | NA | 2 | ||
| Mc C25 |
62 | Right lung cancer, pancoast syndrome | Thoracic, neck and arm | 0.08 | 2 | 8.5 | Stabbing, burning, shooting | N | 3 | NA | 2 |
Notes:
Pain judged to be cancer-independent;
pain also related to surgery and cancer;
pain also related to radiotherapy. Allodynia severity rating: 0 = no pain or discomfort to touch, 1 = uncomfortable, but tolerable to touch, 2 = painful, 3 = extremely painful, patient cannot stand touching; duration of pain was converted to years, the term “months” was set to <1 year in the table and to 0.5 years for calculation of means.
Abbreviations: CGIC, Clinical Global Impression of Change rating during treatment with 5% lidocaine-medicated plaster: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change; CRPS, complex regional pain syndrome; F, female; M, male; NA, not available; N, no; X, symptom present; Y, yes.
Table 4.
Case reports for cancer pain with neuropathic components related to cancer (pain was additionally related to radiotherapy in two patients, to surgery in two patients, and to chemotherapy and surgery in one patient)
| Gender/case number | Age | Primary diagnosis (pain indication/cause of pain) | Localization of pain symptoms | Duration of pain in years | Hyper-algesia | Allodynia/allodynia severity rating | Pain intensity (0–10) | Pain quality | Mono-therapy | Plasters per day | Duration of plaster treatment (months) | CGIC score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M C10 |
52 | Nociceptive + neuropathic pain/cancer pain/infiltration of the brachial plexus | Neck and arm (right) | 4 | 5–7 | Stabbing, burning, shooting, dullness, itch, sensory deficit | N | NA | NA | 1 | ||
| M C37 |
80 | Neuropathic pain, lung neoplasia | Dorsal | 5 | 3 | 7 | Burning, like water falling | N | 1 | 12 | 1 | |
| F C40 |
82 | Bone metastases, colon neoplasia | Dorsal | 10 | X | 2 | 8 | Burning | N | 1 | 18 | 1 |
| F C33 |
68 | Breast adenocarcinoma, bone metastasis T11 and L1 | Dorsolumbar with intercostal irradiation | 0.06 | X | 6 | Shooting, like raging toothache | N | 1/4 daily for 1st few days then every 2nd day | 0.75 | 1 | |
| M C17 |
71 | Neoplastic pain | Right hip | 0.67 | 1 | 7 | Burning | Y | 1 | 4 | 2 | |
| M C34 |
69 | NSCLC right middle lobe | Lombar | 0.02 | X | 7 | Burning, shooting | N | 1/2 | 2 | 2 | |
| M C36 |
69 | Bronchogenic cancer (right IIIb → IV) | Scapulalgia | <0.08 | X | NA | Shooting | N | 1/4 | 3 | 2 | |
| F C15 |
47 | Uterus sarcoma, intra/retroperitoneal filiae | Left lower leg | 0.25 | 3 | 8 | Stabbing, burning | N | 2 | 2 | 2 | |
| Fa C1 |
54 | Meningioma of right cavernous sinus with oculomotor failure (III and IV nerve paralysis) and loss of visual acuity | Right frontoparietal headache (hemicrania) | 7 | X | 2 | 2–3 | Stabbing, burning, shooting | N | 1 | 18 | 2 |
| Mb C29 |
42 | Carcinoma left kidney | Region of postoperative scar | 0.007 | X | 2 | 9 | Stabbing, burning | N | 1 | 1 | 2 |
| F C12 |
51 | Cancer nociceptive + neuropathic (sigmoide) | Belly, thigh, leg | 0.25 | 2 | 7 | Stabbing, burning, shooting, tightness | N | NA | 1 | 2 | |
| F C14 |
48 | Breast cancer left, pulmonal filiae + bone | Left calf due to cancer infiltration of sacrum and S1 root | 0.08 | 1 | 7 | Burning | N | 1 | >2 | 2 | |
| Mb,c C8 |
68 | Planoepithelial carcinoma right lung (complete resection and chemo) | Thoracic region (scar) | 0.5 | 1 | 6 | Shooting | Y | 1 | 0.5 | 2 | |
| Fb C2 |
70 | Liposarcoma of left kidney | Left psoas + T12 | 1 | X | 2 | 3–4 | Dysesthesia | N | 2 | 16 | 3 |
| F C11 |
46 | Cancer pain metastasis/vertebral T4 | Thoracic | 10 | 6–7 | Stabbing, burning, shooting | N | 3 | 4 | 3 | ||
| F C9 |
56 | Cancer pain nociceptive and neuropathic | Cervicalgies, rachialgies, cruralgies | 2 | 7 | Burning | N | NA | NA | 3 | ||
| F C22 |
89 | Metastases in vertebral column | Thoracic spine, radiation to the anterior thoracic wall | 0.5 | X | 6 | Stabbing, shooting, numbness in anterior thoracic wall | N | 1 | 1 | 3 | |
| Ma C24 |
53 | Lung cancer, right upper lobectomy | Right thoracic pain post cancer surgery | 0.5 | X | 1 | 5 | Burning, shooting | N | 3 | NA | 4 |
| M C30 |
74 | Prostate carcinoma, bone metastases, stenosis spinalis | Lumbar spine – level L1–L2 | 0.08 | X | 1 | 9 | Burning, shooting | N | 1 | 0.5 | 4 |
| F C31 |
55 | Bone metastases from breast cancer, radiotherapy | Interscapular pain | 0.17 | X | 1 | 8 | Burning | N | 1 | 0.4 | 4 |
| M C35 |
69 | Adenocarcinoma, right lung superior lobe | Axillar | <0.08 | 3 | 7 | Shooting | N | 1/2 | 0.1 | 4 |
Notes:
Pain also related to radiotherapy;
pain also related to surgery;
pain also related to chemotherapy. Allodynia severity rating: 0 = no pain or discomfort to touch, 1 = uncomfortable, but tolerable to touch, 2 = painful, 3 = extremely painful, patient cannot stand touching; duration of pain was converted to years, the term “weeks” was set to <0.08 in the table and to 0.05 for calculation of means.
Abbreviations: CGIC, Clinical Global Impression of Change rating during treatment with 5% lidocaine-medicated plaster: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change; F, female; M, male; NA, not available; N, no; NSCLC, non-small-cell lung carcinoma; X, symptom present; Y, yes.
Pain related to chemotherapy
Pain was considered related to chemotherapy in seven patients (Table 1). Four males and three females (age range 43–68 years, pain duration 1 month–1.67 years) received lidocaine plaster treatment for 0.5–2 months as monotherapy (three patients) or add-on treatment (four patients). Treatment success was reported for all seven patients (CGIC ≤ 2). Complex regional pain syndrome was diagnosed in two of these patients and one patient presented with thoracic scar pain (considered related to chemotherapy, surgery and cancer).
Example cases
A 60-year-old male with lung adenocarcinoma whose neuropathic, burning pain in his right foot and thoracotomy scar was considered related to chemotherapy and had been present for 18 months. The average pain intensity varied from 4 to 9 on the visual analog scale (VAS) following activity but also with breakthrough pain. He also presented with hyperalgesia und uncomfortable allodynia (severity score 1). Prior unsuccessful treatments included pregabalin, carbamazepine, and paracetamol. The patient received three lidocaine plasters daily concomitant to gabapentin (600 mg three times a day) for 2 months and was rated much improved.
A 48-year-old female with ovarian cancer whose stabbing, neuropathic pain in her swollen hands (mainly right hand) was considered related to chemotherapy and had been present for 3 months; she was diagnosed with CRPS I 2 months after chemotherapy. She also presented with hyperalgesia and painful allodynia (severity score 2) and had received 50 μg/hour fentanyl patch as previous treatment. The average pain intensity was 7 on the numerical rating scale. The patient received two lidocaine plasters daily concomitant to amitriptyline (25 mg three times a day) for 2 weeks and was rated much improved, with no more pain or edemas and improved functionality of her hands.
Pain related to radiotherapy
Table 2 lists the cases of neuropathic pain considered related to radiotherapy. Eight of the nine patients (five females/four males, age range 42–68 years, pain duration 3 weeks–10 years) received lidocaine plaster as add-on therapy. Six patients were at least much improved by the treatment; minimal or no improvement was documented for the other three patients. As neuropathic pain was judged to be related also to surgery in four patients, to cancer in another two, and to chemotherapy in one patient, it is difficult to ascertain the role of radiotherapy as a potential outcome predictor in this small sample size.
Table 2.
Case reports for cancer pain with neuropathic components related to radiotherapy (pain was additionally related to surgery in 4 patients, to cancer in 2 patient and to chemotherapy in one patient)
| Gender /case number | Age | Primary diagnosis (pain indication/cause of pain) | Localization of pain symptoms | Duration of pain in years | Hyper-algesia | Allodynia/ allodynia severity rating | Pain intensity (0–10) | Pain quality | Mono-therapy | Plasters per day | Duration of plaster treatment (months) | CGIC score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ma C3 |
42 | Left leg fusiform cell sarcoma | Leg – knee | 2 | X | 4–5 | Burning, like an electrical shock | N | 1 | 8 | 1 | |
| F C4 |
65 | Uterus adenocarcinoma | Feet, legs | 10 | X | 2 | 3–4 | Burning | Y | 1 | 3 | 1 |
| Fb C1 |
54 | Meningioma of right cavernous sinus with oculomotor failure (III and IV nerve paralysis) and loss of visual acuity | Right frontoparietal headache (hemicrania) | 7 | X | 2 | 2–3 | Stabbing, burning, shooting | N | 1 | 18 | 2 |
| Mc C25 |
62 | Right lung cancer, pancoast syndrome | Thoracic, neck and arm | 0.08 | 2 | 8.5 | Stabbing, burning, shooting | N | 3 | NA | 2 | |
| F C27 |
68 | Mesothelioma | Arm and breast | 0.25 | 3 | 8 | Burning | N | 3 | NA | 2 | |
| Ma C28 |
53 | Cavum and sinus piriformis carcinoma | Right part of neck | 0.33 | X | 2 | 7 | Burning, shooting | N | 2 | NA | 2 |
| Fa C26 |
44 | Occipital sarcoma, epidural metastasis, L3 L4 L5 metastases | L3 radicular pain | 0.06 | 6 | N | 3 | NA | 3 | |||
| Mb C24 |
53 | Lung cancer, right upper lobectomy | Right thoracic pain post cancer surgery | 0.5 | X | 1 | 5 | Burning, shooting | N | 3 | NA | 4 |
| Fa C20 |
67 | Persistent postmastectomy pain | Chest wall, right side | 4 | 2 | 8 | Burning, shooting | N | 1 | 3 | 4 |
Notes:
Pain also related to surgery;
pain also related to cancer;
pain also related to chemotherapy. Allodynia severity rating: 0 = no pain or discomfort to touch, 1 = uncomfortable, but tolerable to touch, 2 = painful, 3 = extremely painful, patient cannot stand touching; duration of pain was converted to years.
Abbreviations: CGIC, Clinical Global Impression of Change rating during treatment with 5% lidocaine-medicated plaster: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change; F, female; M, male; NA, not available; N, no; X, symptom present; Y, yes.
Pain related to surgery
Fifteen cases of cancer pain with neuropathic components (eight females/seven males, age range 42–79 years, pain duration 10 days–4 years) were considered related to surgery (Table 3). Lidocaine plaster was mainly administered as add-on therapy (73.3% of the patients) and resulted in at least much improvement for eleven patients (73.3%), minimal improvement for three patients, and no change for one patient. Concomitant pain medications were reduced in eight of the ten cases with available information. Neuropathic pain was also related to radiotherapy in four patients, cancer in two, and cancer and chemotherapy in one patient. Seven (87.5%) of the eight patients in whom neuropathic pain was considered to be only related to surgery reported at least much improvement and one patient an unchanged condition. For four of these improved patients, dose reductions in concomitant pain medications were documented.
Table 3.
Case reports for cancer pain with neuropathic components related to surgery (pain was additionally related to radiotherapy in 4 patients, to cancer in 2 patients, and to chemotherapy and cancer in 1 patient)
| Gender /case number | Age | Primary diagnosis (pain indication/cause of pain) | Localization of pain symptoms | Duration of pain in years | Hyper-algesia | Allodynia/ allodynia severity rating | Pain intensity (0–10) | Pain quality | Mono-therapy | Plasters per day | Duration of plaster treatment (months) | CGIC score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ma C3 |
42 | Left leg fusiform cell sarcoma | Leg – knee | 2 | X | 4–5 | Burning, like an electrical shock | N | 1 | 8 | 1 | |
| F C32 |
44 | Right mastectomy and mammae prosthesis | Axilla and inner part of forearm | 2.5 | 1 | 7 | Burning | Y | 1 every second day | 0.25 | 1 | |
| F C19 |
74 | Postmastectomy | Thorax (scar pain) | 1.08 | X | 2 | 7–8 | Burning, like electric discharge | N | 1/2 | 24 (interrupted) | 1 |
| F C23 |
58 | Postthoracotomy pain | Posterolateral (left) +/− 7th rib | 0.5 | 2 | 7 | Shooting | Y | 1/2 | 4 | 1 | |
| M C16 |
79 | Rhabdomyosarcoma thorax surgery (+latissimus plastic reconstruction) | Lateral thorax left | 0.33 | 1 (ventral scar), 2 (dorsal scar) | 9 | Stabbing, shooting | N | 2 | 5 | 2 | |
| F C38 |
60 | Mastectomy | Scar of breast surgery | 3 | X | 3 | 9 | Burning, shooting | N | 1 | 18 | 2 |
| Mb C29 |
42 | Carcinoma left kidney | Region of postoperative scar | 0.007 | X | 2 | 9 | Stabbing, burning | N | 1 | 1 | 2 |
| M C39 |
56 | Thoracic surgery for lung cancer | Dorsal (localization of chest drain) | 0.5 | X | 1 | 6 | Shooting | Y | 1/2 | 2 | 2 |
| F C13 |
43 | Nociceptive + neuropathic pain posthysterectomy | Vaginal + inguinal | 0.08 | X | NA | Burning | N | 2 | NA | 2 | |
| Ma C28 |
53 | Cavum and sinus piriformis carcinoma | Right part of neck | 0.33 | X | 2 | 7 | Burning, shooting | N | 2 | NA | 2 |
| Mb,c C8 |
68 | Planoepithelial carcinoma right lung (complete resection and chemo) | Thoracic region (scar) | 0.5 | 1 | 6 | Shooting | Y | 1 | 0.5 | 2 | |
| Fb C2 |
70 | Liposarcoma of left kidney | Left psoas + T12 nerve root | 1 | X | 2 | 3–4 | Dysesthesia | N | 2 | 16 | 3 |
| M C21 |
42 | Pulmonary cancer-persistent pain Postthoracotomy | Right side of chest, radiation to scapula | 1 | X | 2 | 4 | Shooting | N | 1 | 2 | 3 |
| Fa C26 |
44 | Occipital sarcoma, epidural metastasis, L3 L4 L5 metastases | L3 radicular pain | 0.06 | 6 | N | 3 | NA | 3 | |||
| Fa C20 |
67 | Persistent postmastectomy pain | Chest wall, right side | 4 | 2 | 8 | Burning, shooting | N | 1 | 3 | 4 |
Notes:
Pain also related to radiotherapy;
pain also related to cancer;
pain also related to chemotherapy. Allodynia severity rating: 0 = no pain or discomfort to touch, 1 = uncomfortable, but tolerable to touch, 2 = painful, 3 = extremely painful, patient cannot stand touching; duration of pain was converted to years.
Abbreviations: CGIC, Clinical Global Impression of Change rating during treatment with 5% lidocaine-medicated plaster: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change; F, female; M, male; NA, not available; N, no; X, symptom present; Y, yes.
Add-on treatment with lidocaine plaster led to much improvement in a 60-year-old patient presenting with scar pain of 3 years’ duration following mastectomy. Accompanying symptoms were hyperalgesia, extremely painful allodynia, and burning and shooting pain. In contrast, the only patient in the surgery group showing no change in pain condition following lidocaine plaster treatment was also a postmastectomy patient. This 67-year-old patient presented with painful allodynia, burning and shooting pain but no hyperalgesia, and her pain condition was also considered related to radiotherapy.
In two of the three cases with minimal improvement, spinal nerves were involved (T12 nerve root and L3 radicular pain). The L3 radicular pain patient showed none of the typical neuropathic pain symptoms, such as hyperalgesia, allodynia, or burning pain. Thus it might be hypothesized that deep and radicular pain might not respond to lidocaine plaster treatment.
Pain related to the cancer
Table 4 lists the 21 neuropathic pain cases related to the tumor (eleven females/ten males, age range 42–89 years, pain duration 10 days–10 years). Only two patients received the lidocaine plaster as monotherapy. Concomitant pain medications were reduced in eight of 14 cases with available information. Pain was also considered to be possibly related to radiotherapy (two patients), surgery (two patients), and surgery and chemotherapy (one patient). Pain was rated at least much improved for 61.9% of the patients; 38.1% showed minimal or no improvement. Ten (62.5%) of the 16 patients in whom neuropathic pain was considered to be related only to the cancer but not to its treatment showed at least much improvement.
Example cases
Lidocaine plaster treatment was successful in an 80-year-old male with lung neoplasia who presented with dorsal neuropathic pain. Pain duration was 5 years, with an average pain intensity of 7 on the numerical rating scale. Clinical symptoms included extremely painful allodynia, burning pain, and pain “like water falling.” He had previously been treated with amitriptyline (50 mg/day). One daily lidocaine plaster was added to his current medication of tramadol (2 × 50 mg/day). During the 12 months of treatment, tramadol intake could be reduced, and the patient was rated very much improved (CGIC = 1).
Possible neuropathic pain did not improve with lidocaine plaster treatment in a 55-year-old white female with bone metastases from breast cancer who had received both chemotherapy and radiotherapy over the last 5 years. The patient was also diagnosed with depression. Interscapular pain had been present for 2 months, with an average intensity of 8 on the VAS. Hyperalgesia, burning pain, and allodynia (severity score = 1) were also documented. Prior therapy included oxycodone (2 × 30 mg/day), diclofenac (2 × 75 mg/day), and morphine sulfate (2 × 20 mg/day). The patient received one daily lidocaine plaster for 12 days in addition to a fentanyl patch (50 μg/hour).
Duration of cancer pain was comparable when stratified according to treatment success: patients with at least much improvement (CGIC ≤ 2) had a pain duration of 1.78 ± 2.78 years, and patients with minimal improvements/no change (CGIC 3 or 4) had suffered pain for 1.76 ± 2.97 years.
Trigeminal neuropathic pain
Twenty-four case reports (62.5% female) were available for review (Figure 2). The mean age of the patients was 61.6 ± 16.2 years, with a mean duration of pain before treatment with the lidocaine plaster of 5.3 ± 6.3 years. The majority of patients (79.2%) received concomitant pain medication during lidocaine plaster treatment. Carbamazepine (currently the treatment of choice for trigeminal neuralgia37) or its prodrug oxcarbazepine were administered concomitantly to six patients. Some patients reported continuous pain, and are therefore probably so-called trigeminal neuralgia type 2, which has been defined by neurosurgeons as pain in which there is prolonged pain 50% of the time.44 Dose reductions of concomitant medication were documented for eight (47.1%) of the 17 cases with available information for this parameter; all were considered at least much improved. Half of the patients (54.2%) were rated at least much improved by lidocaine plaster treatment, and 16.7% minimally improved. There was no change in 25%, and one patient was judged as minimally worse. Two of the patients with no change reported difficulties with plaster attachment to the skin.
Figure 2.
Twenty-four case reports of patients with trigeminal neuropathic pain were available for review.
Abbreviation: CGIC, Clinical Global Impression of Change.
Pain was judged to be predominantly extraoral in the majority of patients (79.2%) and initiated by triggers in 87.5%. Half of the patients with predominantly extraoral pain (57.9%) were at least much improved by the treatment. The pain was described as continuous for 25% of the patients, as continuous with intermittent flares for 45.8%, and as intermittent for 29.2%. Of the 17 patients with either continuous pain or continuous pain with intermittent flares, nine (52.9%) were at least much improved, three (17.7%) minimally improved, and five (29.4%) reported no change in condition. The seven patients with intermittent pain all had a primary diagnosis of trigeminal neuralgia, and six of them had received carbamazepine as prior or concomitant medication. At least much improvement was observed in four patients (57.1%), one improved minimally, one was minimally worse, and there was no change in another.
Duration of trigeminal pain was 4.1 ± 5.6 years for patients with CGIC ≤ 2 and 6.7 ± 7.0 years with a CGIC ≥ 3. Hyperalgesia and stabbing pain were present in only a few patients (four cases with hyperalgesia and six with stabbing pain), burning pain was observed in 13 patients (54.2%), and shooting pain in twelve patients (50%). All four patients with hyperalgesia (100%) were considered at least much improved by lidocaine plaster treatment. Treatment outcome for burning and shooting pain was similar in patients who reported the parameter and in patients who did not. At least much improvement was documented for 53.8% of the patients with burning pain but also for 54.5% of the patients without this type of pain. A similar result was obtained for shooting pain (46.7% with and 41.7% without). Allodynia was present in 20 patients (83.3%); 50% of these patients were considered at least much improved following plaster treatment. A CGIC of 2 was, however, also reported for three of the four patients without allodynia. Eleven patients suffered from severe allodynia (painful or extremely painful); seven of them (63.6%) were at least much improved, two minimally, and there was no change in two other patients. Of the nine patients with mild allodynia, three had a CGIC of 2 and six had a CGIC ≥ 3.
Although neither predominantly extraoral pain nor continuous pain could be regarded as positive outcome predictors according to the case reports, the participating physicians considered both parameters as possible candidates based on their clinical experience. They also suggested PHN as a cause of trigeminal neuropathic pain and type 2 trigeminal neuralgia, ie, with burning pain and allodynia, and traumatic and postsurgical neuropathy as positive predictors. Predominantly intraoral pain was considered as a negative-outcome predictor.
All cases were further stratified according to information about the development of trigeminal neuropathic pain (Table 5).
Table 5.
Case reports for trigeminal pain
| Gender /case number | Age | Primary diagnosis (pain indication/cause of pain/type of pain) | Localization of pain symptoms/predominantly intra-or extraoral | Duration of pain in years | Hyper-algesia | Allodynia/allodynia severity rating | Pain intensity (0–10) | Characteristics of pain | Mono-therapy | Plasters per day | Duration of plaster treatment (months) | CGIC score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigeminal neuralgia | ||||||||||||
| M T4 |
76 | Trigeminal neuralgia Intermittent pain | Left V1 Extraoral |
0.19 | X | 2 | 9 | Stabbing | N | 1/8 | 1.17 | 1 |
| Ma T26 |
82 | Trigeminal neuralgia type 2 Continuous pain | Right V1,2,3 Extraoral |
20 | X | 3 | 8–10 | Burning | N | 1/2 | 0.93 | 1 |
| M T27 |
53 | Trigeminal neuralgia Intermittent pain | Right V Extraoral |
3 | X | 2 | 5 | Stabbing | Y | 1/2 | 0.93 | 1 |
| F T2 |
62 | Trigeminal neuralgia type 2 Continuous pain with intermittent flares | Left Extraoral |
3 | 7 | Burning, shooting | N | 1/2 | NA | 2 | ||
| F T6 |
29 | Trigeminal neuralgia Intermittent pain | V2 Extraoral |
0.5 | X | 1 | 7 | Shooting | N | 1/2 | 0.75 | 2 |
| Ma T24 |
35 | Trigeminal neuralgia Intermittent pain | Left V2 Extraoral |
3 | 2 | 8 | Shooting | N | 1/4 | 3 | 2 | |
| F T18 |
62 | Trigeminal neuralgia type 2 Continuous pain with intermittent flares | Right V2,3 Extraoral, mainly intraoral |
14 | 2 | 8 | Stabbing, shooting, aching | N | 1/2 | 0.25 | 4 | |
| M T1 |
75 | Trigeminal neuralgia Intermittent pain | Left V2,3 Extraoral |
5 | 8 | Stabbing, shooting | N | 1/2 | 2 | 4 | ||
| M T3 |
80 | Trigeminal neuralgia Intermittent pain | Left V2,3 | ≥5 | 1 | 9 | Stabbing, shooting | Y | 1/2, not daily | <0.03 | 5 | |
| F T23 |
82 | Neuralgia SUNCT Continuous pain with intermittent flares | Left V1,2,3 Extraoral |
5 | 1 | 7 | Burning, sharp, aching, dull | N | 1 | 1 | 4 | |
| Trigeminal neuropathic pain following surgery for trigeminal neuralgia | ||||||||||||
| F T17 |
53 | Neuropathic pain Continuous pain | Right V2,3 Intra- and extraoral |
12 | 2 | 10 | Shooting, sharp | Y | 1/2 | 9 | 1 | |
| F T21 |
49 | Neuropathic pain Continuous pain | Right V2,3 Extraoral |
2 | 2 | 8 | Burning | N | 1 weekly | 1 | 3 | |
| F T11 |
64 | Bilateral neuropathic pain Intermittent pain | Right V2,3 Extraoral |
3 | 1 | 8 | Shooting, hot, pricking | N | 1/2 | 0.17 | 3, sleep improved | |
| Fa T7 |
35 | Neuropathic pain Continuous pain with intermittent flares | V2,3 Extraoral | 2 | 2 | 8 | Burning, shooting | N | 1/2 | 0.7 | 3 | |
| F T12 |
72 | Dysesthesia Continuous pain with intermittent flares | Left V3 Extraoral |
7 | 2 | 8 | Heavy, gripping | N | 1/2 | 0.25 | 4 | |
| M T16 |
75 | Dysesthesia Continuous pain | Left V2,3 Extraoral |
25 | 1 | 8 | Burning, soreness, nagging, numbness | Y | 1 | 3 | 4 | |
| Trigeminal neuropathic pain following trauma | ||||||||||||
| F T13 |
50 | Trigeminal posttraumatic neuropathic pain Continuous pain | Left V2 Extraoral |
1.5 | 2 | 9 | Burning, tingling, pricking | N | 1/2 | 4 | 2 | |
| F T22 |
65 | Trigeminal neuropathic pain Continuous pain with intermittent flares | Left V1,2,3 Extraoral |
3 | 1 | 8 | Burning, shooting, throbbing, drilling, heavy, piercing | N | 1 every 2nd day |
7 | 2 sleeps better | |
| F T15 |
63 | Neuropathic pain after extractions Continuous pain with intermittent flares | Right V3 Intraoral Extraoral |
3 | 7 | Shooting, cutting, aching, throbbing | N | 1/2 | 3 | 2 | ||
| M T20 |
44 | Trigeminal neuropathic pain after dental extractions Continuous pain with intermittent flares | Right V1,2,3 Extraoral |
4 | 1 | Burning, sharp, dull | N | 1 every 2nd day |
0.5 | 4 | ||
| Trigeminal neuropathic pain following postherpetic neuralgia | ||||||||||||
| F T10 |
45 | Postherpetic neuralgia Continuous pain with intermittent flares | Right V1 Extraoral |
2.5 | 1 | 10 | Stabbing, searing, burning, shooting, dull | N | 1/2 | 3 | 2 | |
| M T8 |
78 | Postherpetic neuralgia Continuous pain with intermittent flares | Right V1 Extraoral |
0.25 | 2 | 7 | Burning | N | 1/4 | 3 | 2 | |
| F T14 |
74 | Postherpetic neuralgia Continuous pain | Left V1 Extraoral |
1.67 | 4 | Burning, itchy, hot, numb, tingling | Y | Intermittent | 4 | 2 | ||
| F T19 |
76 | Postherpetic neuralgia pain and burning mouth syndrome Continuous pain with intermittent flares | Right V2 Intra- and extraoral |
2 | 1 | 9 | Burning, drilling | N | 1/2 | Few weeks | 3 | |
Notes: Pain was initiated by triggers if not otherwise stated.
no triggers. Allodynia severity rating: 0 = no pain or discomfort to touch, 1 = uncomfortable, but tolerable to touch, 2 = painful, 3 = extremely painful, patient cannot stand touching; duration of pain was converted to years, term “many years” was set to ≥5 years in the table and to 5 years for calculation of means.
Abbreviations: CGIC, Clinical Global Impression of Change rating during treatment with 5% lidocaine-medicated plaster: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse; F, female; M, male; NA, not available; N, no; SUNCT, short unilateral neuralgiform pain with conjunctival tearing; V1, ophthalmic; V2, maxillary; V3, mandibular; X, symptom present; Y, yes.
Idiopathic trigeminal neuropathic pain
Trigeminal neuropathic pain was considered idiopathic in nine patients: six male and three female patients (age range 29–82 years, pain duration 35 days–20 years) with predominantly extraoral pain (77.8%) received lidocaine plaster treatment mainly as add-on treatment. Primary diagnosis was trigeminal neuralgia, which was considered atypical in one case. Six of the patients (66.7%) were considered at least much improved; there was no change in two patients, and one was considered minimally worse.
Example cases
Lidocaine plaster treatment was successful in a 53-year-old male patient who reported intermittent, predominantly extraoral stabbing pain for the right side of his face. The pain had been present for 3 years, with an average pain intensity of 5 on the VAS. He also presented with hyperalgesia and painful allodynia. Previous medications included carbamazepine and pregabalin. After 28 days of treatment with half a lidocaine plaster as monotherapy, the patient’s condition was judged very much improved.
In contrast, 2 months of daily treatment with half a lidocaine plaster in addition to 900 mg carbamazepine twice a day did not change stabbing, shooting, intermittent, predominantly extraoral pain in the left cheek/mandibular of a 75-year-old male patient. His pain had been present for 5 years, with an average pain intensity of 8 on the VAS.
Trigeminal neuropathic pain following surgery for trigeminal neuralgia
Six patients suffered from trigeminal neuropathic pain related to surgery for trigeminal neuralgia (Table 5; five females, one male; age range 35–75 years, pain duration 2–25 years). Treatment with lidocaine plaster varied from 0.17 to 9 months; two patients received the plaster as monotherapy. One patient was rated very much improved, three were minimally improved, and there was no change in another two patients.
Example cases
Treatment was successful in a 53-year-old female patient with atypical trigeminal neuralgia who presented with continuous neuropathic pain (intra- and extraoral) at the right trigeminal nerve divisions 2 and 3. Pain duration was 12 years, with an average pain intensity of 10 on the Brief Pain Inventory. Clinical symptoms included painful allodynia, and shooting and sharp pain. She had previously received carbamazepine, but cannot tolerate systemic medications. Nightly lidocaine plaster treatment (half a plaster) as long-term monotherapy (9 months) very much improved her pain and general status (sleeps well and depression has lifted). In contrast, there was no change following 1 month of lidocaine plaster treatment as add-on therapy to oxcarbazepine 450 mg daily in an 82-year-old female patient also presenting with atypical trigeminal neuralgia. Her extraoral, continuous pain with intermittent flare-ups of the left ophthalmic, maxillary, and mandibular trigeminal branches had been present for 5 years and was considered to have a possible neuropathic component. Burning pain, aching dull pain, acute sharp pain, and allodynia (severity score = 1) were documented. Previous medications included morphine, fentanyl patches, paracetamol/codeine combination and gabapentin; she also received radiofrequency thermocoagulation therapy. Her diagnosis was later changed to short unilateral neuralgiform pain with conjunctival tearing, and she responded to lamotrigine (listed under trigeminal neuralgia in Table 5).
Trigeminal neuropathic pain related to trauma
Trigeminal neuropathic pain was considered related to trauma in four patients (Table 5; three female, one male; age range 44–65 years, pain duration 1.5–4 years). Three patients had predominantly extraoral pain. All patients reported continuous pain (three also with intermittent flares), and three patients had allodynia. Add-on treatment with the lidocaine plaster for 2 weeks to 7 months much improved pain in three cases; there was no change in the one patient receiving the plaster for only 2 weeks.
Trigeminal neuropathic pain following PHN
PHN was considered the cause of trigeminal neuropathic pain in four patients (three female, one male; age range 45–78 years, pain duration 0.25–2.5 years; Table 5). All reported continuous pain (three with intermittent flares), which was predominantly extraoral in three cases. A neuropathic component to the pain was considered definite for all four cases. All patients reported burning pain; three patients had allodynia. Three patients received daily plasters (quarter or half) as additional treatment; one was treated intermittently with the plaster as monotherapy. Treatment much improved pain in the three patients with predominantly extraoral pain (including the patient on monotherapy) and minimally in the fourth patient with intra- and extraoral pain who had only been on lidocaine plaster treatment for a few weeks.
Practical experiences with the 5% lidocaine-medicated plaster were in general positive due to its convenience. Nevertheless, some patients using the lidocaine plaster reported difficulties (numbers in brackets refer to case numbers in Table 5). A female patient (T18) with both intraoral and extraoral pain (trigeminal neuralgia type 2) considered the plaster as not very helpful as her main trigger was eating; she also found the plaster too painful to put on and too fiddly to handle and only used it for 1 week. One patient found the plaster unpleasant to wear and painful to remove (T3). One patient had difficulties in cutting it into smaller pieces and perceived it as conspicuous when wearing it on the face, although the patient would wear it when going out in the cold (T11). Two patients found it difficult to attach to the face (T12 and T16), and one difficult to readjust to the return of pain after removal of plaster (T19). One patient (T8) reported some skin irritation. One patient (T10) reported gaining more benefit from lidocaine plaster after a few weeks of use, which corresponds to a lidocaine plaster study in PHN where a trial period of 2–4 weeks is considered necessary in order to decide whether or not a patient will respond to the treatment.11 Add-on treatment with lidocaine plaster improved sleep in eight patients (T10, T11, T13, T14, T15, T17, T19, and T22) whose pain was often provoked by contact with bedclothes. Regular use of lidocaine plaster in one patient who could not tolerate any systemic medications (T17) completely resolved her depression as measured on the hospital anxiety and depression scale pre- and postuse of the plaster.
Discussion
Evaluation of potential predictors for treatment success with the lidocaine plaster included a number of different parameters specific to the nature of the disorder. Results are therefore discussed separately for each indication; a summary of the overall experience with the plaster is given at the end of the Discussion section.
Cancer pain with neuropathic components
Group discussions and case-report analysis identified some parameters as positive- or negative-outcome predictors for the treatment of neuropathic cancer pain with the 5% lidocaine-medicated plaster, but were not conclusive for several others (Table 6). Not only typical neuropathic pain features, such as hyperalgesia, allodynia, and burning pain, had to be evaluated but also the cause of the neuropathic pain (related to cancer treatment or to the cancer itself) had to be taken into account. Interpretations were difficult when multiple causes for neuropathic pain were present.
Table 6.
Probability of treatment success with 5% lidocaine-medicated plaster
| Probability | Potential predictors | Indication | |
|---|---|---|---|
|
|
|||
| Long-terma | Short-termb | ||
| High | Localized pain | Postherpetic neuralgia | Post herpes zoster |
| Superficial pain | Diabetic polyneuropathy | Scar pain (postsurgery) | |
| Allodynia | Chronic postsurgical pain | ||
| Complex regional pain syndrome | |||
| Medium | Hyperalgesia | Low back pain (chronic) | Low back pain |
| Burning | Cancer pain with neuropathic components | Carpal tunnel syndrome | |
| Stabbing | Trigeminal neuropathic pain | ||
| Shooting | |||
| Numeric rating scale score | |||
| Low | Deep pain | Central pain | Fibromyalgia |
| Numbness | Arthrosis | ||
| Radiating pain | Gout | ||
| Radicular pain | Phantom limb pain | ||
| Heavy sweating | Muscular pain | ||
| Pain site distant from nerve damage | |||
| Chronic widespread | |||
Notes:
Duration months to years;
duration days to weeks.
There had been general agreement in the last three pain specialist meetings that allodynia is a highly probable positive predictor for the indications DPN, CRPS, chronic low back pain with neuropathic components, and chronic neuropathic pain after surgical and nonsurgical trauma.24 Furthermore, marked improvements of allodynia with the lidocaine plaster had been noted in several randomized, controlled studies,9,10,12 and add-on therapy was more often successful in patients with allodynia than in patients without.47 However, the lidocaine plaster was comparably efficacious in patients with painful diabetic polyneuropathy presenting with or without allodynia.48 From their clinical experience, some of the participants in this meeting considered allodynia as a positive outcome predictor for neuropathic cancer pain, while others regarded this parameter as potentially helpful but not sufficient. The analysis of the case reports contributed showed that although there was a slight trend towards a positive outcome in the presence of allodynia or burning pain, and shooting pain had a slightly worse predictive value, 73% of all patients showed much or very much improvement, regardless of any of these parameters being present or not. A similar result was obtained for hyperalgesia. From these results, the presence of allodynia, hyperalgesia, or pain quality does not provide a clear indication of treatment success for neuropathic cancer pain. A possible reason for our findings could be the high incidence of mixed pain conditions in cancer pain.26,27
Chronic postsurgical neuropathic pain is often severely debilitating, affecting the economic and emotional well-being of the patients; incidences vary according to type of surgery.49 Lidocaine plaster treatment was almost always successful in cancer patients whose pain was related to surgery. This finding did not come as a surprise, since it is in agreement with an open-label study by Hans et al15 in patients suffering from neuropathic scar pain and a previous case series analyzed for the 2009 pain-specialist meeting.24
Neuropathy is a common adverse effect of chemotherapy, with tingling, numbness, impaired sensory function, and pain the most common symptoms.50 All seven patients with neuropathic pain considered related to chemotherapy were successfully treated with the lidocaine plaster. This result is encouraging, as chemotherapy-induced peripheral neuropathy has so far been relatively refractory to existing first-line treatments for neuropathic pain.8 In two of the patients, the pain was diagnosed as CPRS, which has been treated successfully with the plaster in previous cases18,51,52 and was given a high probability of treatment success.24
Duration of neuropathic pain and pain directly related to the cancer did not have any predictive value. A possible predictor role for radiotherapy was difficult to ascertain because the neuropathic pain of most of these patients was also related to other causes.
A number of case reports did not include data for dose reductions of concomitant pain medications; the available data indicate that treatment with the lidocaine plaster allowed a dose reduction in at least 50% of the patients using the plaster as adjunct treatment. This finding corresponds to results of other investigations with the lidocaine plaster reporting marked reductions in systemic medication.11,53 A reduction of concomitant systemic pain medications while maintaining efficient pain relief with the lidocaine plaster might lower the risk of systemic side effects, such as nausea, sedation, dizziness, and constipation, and could thus improve quality of life and patient compliance. In addition, the low systemic exposure after plaster application54 might also reduce the risk of pharmacokinetic interactions with concomitant medications in this likely polymedicated patient population.
Trigeminal neuropathic pain
Lidocaine plaster treatment was successful (at least much improved) in approximately every second patient (54%) described in the submitted case reports. Given the small number of case reports (n = 24) investigated for this indication, it is therefore difficult to judge the predictive value of different parameters. Furthermore, the site of the pain (eg, face, intraoral) and the sometimes very small size of the painful area can pose technical problems with plaster application and attachment in this indication. Owing to the high muscle activity and movement in the face, plaster attachment can be difficult, especially if smaller plaster pieces are used. Its appearance might also limit its use during the daytime, when many patients get evoked pain from triggers.
Although there was no clear indication from the case report analysis, participants considered continuous pain and predominantly extraoral pain as possible positive outcome predictors and predominantly intraoral pain as a possible negative predictor.
Signs and symptoms of different types of facial neuropathic pain can overlap considerably.42,43 In 14 of the included case reports, trigeminal neuralgia was stated as the primary diagnosis/cause of pain. Some of these patients might have presented with neuropathic pain caused by trigeminal neuralgia instead of trigeminal neuropathic pain, in which case the lidocaine plaster would not have been the most appropriate treatment. Facial herpes zoster infection and subsequent PHN is not uncommon and appears to affect women more frequently than men.55 In 17% of the current case series, PHN was considered the cause of trigeminal neuropathic pain, and three of the four patients were in fact women; however, the number of cases is too small to draw any conclusions. All PHN cases with predominately extraoral pain showed much improvement, which corresponds to the overall observation that extraoral pain might be a possible positive outcome predictor, in particular if caused by PHN.
Misdiagnosis can lead to a long trial-and-error period to find the right medication and can substantially impair the quality of life of the patient. As an example, we refer the reader to the case of a patient with short unilateral neuralgiform pain with conjunctival tearing that had been misdiagnosed as atypical trigeminal neuralgia for 5 years, as previously described. The implications of both misdiagnosis and potential mismanagement are discussed in a recent review.40
In contrast to cancer pain with neuropathic components, there was no predictive value for the parameter “pain related to surgery” for trigeminal neuropathic pain. It should be noted that the term “pain related to surgery” has a different meaning in the two indications. In cancer patients, it refers to localized scar pain following surgery; in patients with trigeminal neuropathic pain, however, procedures such as dental or trigeminal surgery might trigger a nerve response not localized at the site of the wound.
Pain quality, mild allodynia, and cold allodynia were considered of no predictive value, but the case analysis showed a positive outcome in patients with severe allodynia, hyperalgesia, PHN as the cause of neuropathic pain, and trauma.
Two patients in the current case series (T4 and T6) showed an excellent response to add-on treatment with lidocaine plaster, but the duration of the pain was only 9 weeks and 6 months, respectively. These patients could have been going into remission, which is common after a first attack of trigeminal neuralgia.
Trigeminal neuropathic pain patients used the lidocaine plaster mainly at night when allodynia interfered with sleep, and one-third of the patients did in fact report improved sleep. The recording of sleep patterns pre- and postuse could be considered a future study objective to investigate whether this might be a useful outcome parameter. In general, however, it would be important to consider lidocaine plaster in those patients who are on other drugs that could interfere with systemic analgesic medications; this is for instance highly likely in elderly patients.
It might be generally difficult to justify the continuous application of lidocaine plaster in trigeminal neuropathic pain patients with episodic pain and in patients with predominately evoked pain due to light touch triggers who may find the plaster difficult to apply. Patients with spontaneous, more continuous pain may be more willing to try.
Many of the patients described in these case reports used lidocaine plaster intermittently and possibly not for long enough to have an effect, since data from controlled studies in PHN and DPN suggest a more pronounced effect after 2–4 weeks.56 Thus, administration should be regular and for at least a few weeks.
Successful treatment of different neuropathic pain states with the lidocaine plaster has led to the suggestion of a common symptomatology with common predictors of treatment success. A ranking table proposing treatment success with high, medium, or low probability for a combination of a predictor with an indication was therefore created and updated in each of the four pain-specialist meetings based on published evidence, submitted case reports, and the experience of the participants (Table 6).
Overall, there is a medium probability of treatment success for cancer pain with neuropathic components, which seems to be independent of the typical neuropathic features hyperalgesia, allodynia, and pain qualities such as burning and shooting pain. Treatment benefits, however, seem to be probable if a relationship of the pain to surgery or chemotherapy can be established and if chemotherapy-induced neuropathic pain is a localized pain permitting the use of a plaster. The participants of the meeting did not think that the term “cancer pain” represents a valid pain classification. Pain in a cancer patient may be caused by the tumor itself, may be treatment-related, may be both, or may in fact be tumor-independent. The treating physician’s utmost goal must be to relieve this pain as quickly as possible and therefore avoid long trial-and-error periods by careful diagnostics. In any patient presenting with cancer pain, it needs to be established, if possible, whether the pain is likely of nociceptive or neuropathic nature. Given the results of our case analysis, a trial period with lidocaine plaster (possibly as add-on with subsequent attempts to reduce other medications) is worth pursuing if the pain is considered to be localized neuropathic pain and also likely to be caused by surgery or chemotherapy. Despite the encouraging observation that two-thirds of the radiotherapy patients experienced pronounced pain relief under treatment with the plaster, we cannot draw any conclusions concerning radiotherapy-related neuropathic pain from this case series. There were too many confounding factors, ie, other or additional possible causes for the neuropathic pain. Further study of this condition with a larger sample size is clearly warranted.
The available data did not permit identification of any predictors suggestive of treatment success or treatment failure with lidocaine plaster for the indication trigeminal neuropathic pain. However, since more than half of the patients showed a positive response to plaster treatment, the plaster may be a valuable add-on option for patients insufficiently treated with their present medication, and it may also be considered as monotherapy in patients who are unable to tolerate systemic medication. These assumptions need, however, to be investigated in future studies.
Limitations
It should be noted that the case reports submitted for discussion at the meeting were selected by the participants. The selection is likely biased and thus is not necessarily representative of the entire patient population treated for neuropathic cancer pain or trigeminal neuropathic pain. The small sample size of the different subgroups also needs to be acknowledged. Careful sensory testing with clear indications of the type of sensory deficit experienced, as suggested by Maier et al57 was not carried out in the small sample, and this would need to be done in future larger studies, as it may provide clear predictors for outcome. However, the reports permit a first evaluation of potential benefits of lidocaine plaster treatment for these conditions, and thus support conducting large, well-designed multicenter studies.
Acknowledgments
The authors wish to thank all pain specialists who contributed case reports: AF Chapelle, J Maes (Belgium), M Hakl (Czech Republic), JB Caillet, D Lefebvre Kuntz, J Pouymayou (France), B Kukowski (Germany), M Kocot-Kepska, A Przeklasa-Muszyńska (Poland), S Abreu, A Gomes, T Van Pato (Portugal), H Jakubikova (Slovakia), N Krčevski Škvarč (Slovenia), JM Gómez Argüelles (Spain), J Martina (The Netherlands). Further thanks go to E Grosselindemann (Brett Medical Writing, Australia) and B Brett (Brett Medical Writing, Germany) for writing and editorial assistance and publication coordination.
Footnotes
Disclosure
The meeting was facilitated by Grünenthal GmbH, Aachen, Germany. All costs associated with this publication were met by Grünenthal. Grünenthal were given the opportunity to review and comment on the manuscript; however, the authors take full responsibility for the content. JZ is a consultant for Convergence Pharmaceuticals Ltd on the trial design for a new drug for trigeminal neuralgia and undertook the work at UCL/UCLHT which received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre funding scheme. KUK is a consultant for and/or received speaker fees from Astellas, Berlin Chemie, betapharm Arzneimittel, Boehringer Ingelheim, Eisai, Grünenthal, Lilly, Mundipharma, and Medi Bayreuth. SN received consultancy honoraria or research grants from the following companies in the past 12 months: Johnson and Johnson, Endo Pharmaceuticals, Cephalon, ProStakan, and Covidien. LB received grants from Archemides and Nycomedes in the past 12 months.
References
- 1.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 2.International Association for the Study of Pain. Neuropathic pain. 2012. [Accessed July 5, 2011]. Available from: http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Defi...isplay.cfm&ContentID=1728#Neuropathicpain.
- 3.Smith BH, Torrance N. Epidemiology of neuropathic pain. Pain Manage. 2011;1:87–96. doi: 10.2217/pmt.10.5. [DOI] [PubMed] [Google Scholar]
- 4.Schmader KE, Baron R, Haanpää ML, et al. Treatment considerations for elderly and frail patients with neuropathic pain. Mayo Clin Proc. 2010;85( Suppl 3):S26–S32. doi: 10.4065/mcp.2009.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor AB. Neuropathic pain. Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 7.Finnerup NB, Otto M, Jensen TS, Sindrup SH. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9:36. [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85( Suppl 3):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron R, Mayoral V, Leijon G, et al. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25:1663–1676. doi: 10.1185/03007990903047880. [DOI] [PubMed] [Google Scholar]
- 10.Binder A, Bruxelle J, Rogers P, et al. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post-herpetic neuralgia: results of a double-blind, placebo-controlled, multinational efficacy and safety trial. Clin Drug Investig. 2009;29:393–408. doi: 10.2165/00044011-200929060-00003. [DOI] [PubMed] [Google Scholar]
- 11.Rehm S, Binder A, Baron R. Post-herpetic neuralgia – 5% lidocaine medicated plaster, pregabalin, or a combination of both? A randomized, open, clinical effectiveness study. Curr Med Res Opin. 2010;26:1607–1619. doi: 10.1185/03007995.2010.483675. [DOI] [PubMed] [Google Scholar]
- 12.Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106:151–158. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann DN, Barbano RL, Hart-Gouleau S, Pennella-Vaughan J, Dworkin RH. An open-label study of the lidocaine patch 5% in painful idiopathic sensory polyneuropathy. Pain Med. 2005;6:379–384. doi: 10.1111/j.1526-4637.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 14.Nalamachu S, Crockett RS, Gammaitoni AR, Gould EM. A comparison of the lidocaine patch 5% vs naproxen 500 mg twice daily for the relief of pain associated with carpal tunnel syndrome: a 6-week, randomized, parallel-group study. MedGenMed. 2006;8:33. [PMC free article] [PubMed] [Google Scholar]
- 15.Hans G, Joukes E, Verhulst J, Vercauteren M. Management of neuropathic pain after surgical and non-surgical trauma with lidocaine 5% patches: study of 40 consecutive cases. Curr Med Res Opin. 2009;25:2737–2743. doi: 10.1185/03007990903282297. [DOI] [PubMed] [Google Scholar]
- 16.Galer BS, Gammaitoni AR. More than 7 years of consistent neuropathic pain relief in geriatric patients. Arch Intern Med. 2003;163:628. doi: 10.1001/archinte.163.5.628. [DOI] [PubMed] [Google Scholar]
- 17.Hans G, Sabatowski R, Binder A, et al. Efficacy and tolerability of a 5% lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study. Curr Med Res Opin. 2009;25:1295–1305. doi: 10.1185/03007990902901368. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm IR, Tzabazis A, Likar R, et al. Long-term treatment of neuropathic pain with a 5% lidocaine medicated plaster. Eur J Anaesthesiol. 2010;27:169–173. doi: 10.1097/EJA.0b013e328330e989. [DOI] [PubMed] [Google Scholar]
- 19.Delorme C, Navez ML, Legout V, Deleens R, Moyse D. Treatment of neuropathic pain with 5% lidocaine medicated plaster: five years of clinical experience. Pain Res Manage. 2011;16:259–262. doi: 10.1155/2011/359591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatowski R, Hans G, Tacken I, Kapanadze S, Buchheister B, Baron R. Safety and efficacy outcomes of long-term treatment up to 4 years with 5% lidocaine medicated plaster in patients with post-herpetic neuralgia. Curr Med Res Opin. 2012;28:1337–1346. doi: 10.1185/03007995.2012.707977. [DOI] [PubMed] [Google Scholar]
- 21.Correa-Illanes G, Calderón W, Roa R, Piñeros JL, Dote J, Medina D. Treatment of localized post-traumatic neuropathic pain in scars with 5% lidocaine medicated plaster. Local Reg Anesth. 2010;3:77–83. doi: 10.2147/LRA.S13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustorff B, Hauer D, Thaler J, Seis A, Draxler J. Antihyperalgesic efficacy of 5% lidocaine medicated plaster in capsaicin and sunburn pain models – two randomized, double-blinded, placebo-controlled crossover trials in healthy volunteers. Expert Opin Pharmacother. 2011;12:2781–2790. doi: 10.1517/14656566.2011.601868. [DOI] [PubMed] [Google Scholar]
- 23.Baron R, Tölle TR. Assessment and diagnosis of neuropathic pain. Curr Opin Support Palliat Care. 2008;2:1–8. doi: 10.1097/SPC.0b013e3282f57da5. [DOI] [PubMed] [Google Scholar]
- 24.Nicolaou A, Nicholson B, Hans G, Brasseur L. Outcome predictors for treatment success with 5% lidocaine medicated plaster in low back pain with neuropathic components and neuropathic pain after surgical and nonsurgical trauma. J Pain Res. 2011;4:25–38. doi: 10.2147/JPR.S15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeeney BE. Adjuvant agents in cancer pain. Clin J Pain. 2008;24:S14–S20. doi: 10.1097/AJP.0b013e31816b5976. [DOI] [PubMed] [Google Scholar]
- 26.Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. Pain. 1999;82:263–274. doi: 10.1016/S0304-3959(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 27.Grond S, Radbruch L, Meuser T, Sabatowski R, Loick G, Lehmann KA. Assessment and treatment of neuropathic cancer pain following WHO guidelines. Pain. 1999;79:15–20. doi: 10.1016/S0304-3959(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 28.Jain PN, Chatterjee A, Hom Choudhary A, Sareen R. Prevalence, etiology, and management of neuropathic pain in an Indian cancer hospital. J Pain Palliat Care Pharmacother. 2009;23:114–119. doi: 10.1080/15360280902900489. [DOI] [PubMed] [Google Scholar]
- 29.Bhatnagar S, Mishra S, Roshni S, Gogia V, Khanna S. Neuropathic pain in cancer patients – prevalence and management in a tertiary care anesthesia-run referral clinic based in urban India. J Palliat Med. 2010;13:819–824. doi: 10.1089/jpm.2009.0405. [DOI] [PubMed] [Google Scholar]
- 30.García de Paredes ML, del Moral González F, Martínez del Prado P, et al. First evidence of oncologic neuropathic pain prevalence after screening 8615 cancer patients. Results of the On study. Ann Oncol. 2011;22:924–930. doi: 10.1093/annonc/mdq449. [DOI] [PubMed] [Google Scholar]
- 31.Lema MJ, Foley KM, Hausheer FH. Types and epidemiology of cancer-related neuropathic pain: the intersection of cancer pain and neuropathic pain. Oncologist. 2010;15( Suppl 2):3–8. doi: 10.1634/theoncologist.2009-S505. [DOI] [PubMed] [Google Scholar]
- 32.Urch CE, Dickenson AH. Neuropathic pain in cancer. Eur J Cancer. 2008;44:1091–1096. doi: 10.1016/j.ejca.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Cancer: WHO’s pain ladder. [Accessed July 5, 2011]. Available from: http://www.who.int/cancer/palliative/painladder/en.
- 34.Thapa D, Rastogi V, Ahuja V. Cancer pain management – current status. J Anaesthesiol Clin Pharmacol. 2011;27:162–168. doi: 10.4103/0970-9185.81820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hans GH, Robert DN, Van Maldeghem KN. Treatment of an acute severe central neuropathic pain syndrome by topical application of lidocaine 5% patch: a case report. Spinal Cord. 2008;46:311–313. doi: 10.1038/sj.sc.3102098. [DOI] [PubMed] [Google Scholar]
- 36.Fleming JA, O’Connor BD. Use of lidocaine patches for neuropathic pain in a comprehensive cancer centre. Pain Res Manag. 2009;14:381–388. doi: 10.1155/2009/723179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheville AL, Sloan JA, Northfelt DW, et al. Use of a lidocaine patch in the management of postsurgical neuropathic pain in patients with cancer: a phase III double-blind crossover study (N01CB) Support Care Cancer. 2009;17:451–460. doi: 10.1007/s00520-008-0542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koopman JSHA, Dieleman JP, Huygen FJ, de Mos M, Martin CGM, Sturkenboom MCJM. Incidence of facial pain in the general population. Pain. 2009;147:122–127. doi: 10.1016/j.pain.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Zakrzewska JM, McMillan R. Trigeminal neuralgia: the diagnosis and management of this excruciating and poorly understood facial pain. Postgrad Med J. 2011;87:410–416. doi: 10.1136/pgmj.2009.080473. [DOI] [PubMed] [Google Scholar]
- 40.Zakrzewska JM. Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother. 2010;11:1239–1254. doi: 10.1517/14656561003767449. [DOI] [PubMed] [Google Scholar]
- 41.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd ed. Suppl 1 1. Vol. 24. Cephalalgia: 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 42.Zakrzewska JM. Diagnosis and differential diagnosis of trigeminal neuralgia. Clin J Pain. 2002;18:14–21. doi: 10.1097/00002508-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Forssell H, Tenovuo O, Silvoniemi P, Jääskeläinen SK. Differences and similarities between atypical facial pain and trigeminal neuropathic pain. Neurology. 2007;69:1451–1459. doi: 10.1212/01.wnl.0000277274.83301.c0. [DOI] [PubMed] [Google Scholar]
- 44.Limonadi FM, McCartney S, Burchiel KJ. Design of an artificial neural network for diagnosis of facial pain syndromes. Stereotact Funct Neurosurg. 2006;84:212–220. doi: 10.1159/000095167. [DOI] [PubMed] [Google Scholar]
- 45.Baad-Hansen L. Atypical odontalgia – pathophysiology and clinical management. J Oral Rehab. 2008;35:1–11. doi: 10.1111/j.1365-2842.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 46.Renton T, Yilmaz Z. Managing iatrogenic trigeminal nerve injury: a case series and review of the literature. Int J Oral Maxillofac Surg. 2012;41:629–637. doi: 10.1016/j.ijom.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Kern KU, Kohl M, Kiefer RT. Lidocaine patch for therapy of neuropathic and non-neuropathic pain : a clinical case series of 87 patients. Nervenarzt. 2010;81:1490–1497. doi: 10.1007/s00115-010-3060-2. German. [DOI] [PubMed] [Google Scholar]
- 48.Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004;61:914–918. doi: 10.1001/archneur.61.6.914. [DOI] [PubMed] [Google Scholar]
- 49.Shipton E. Post-surgical neuropathic pain. ANZ J Surg. 2008;78:548–555. doi: 10.1111/j.1445-2197.2008.04569.x. [DOI] [PubMed] [Google Scholar]
- 50.Kautio AL, Haanpää M, Kautiainen H, Kalso E, Saarto T. Burden of chemotherapy-induced neuropathy – a cross-sectional study. Support Care Cancer. 2011;19:1991–1996. doi: 10.1007/s00520-010-1043-2. [DOI] [PubMed] [Google Scholar]
- 51.Frost SG. Treatment of complex regional pain syndrome type 1 in a pediatric patient using the lidocaine patch 5%: a case report. Curr Ther Res. 2003;64:626–629. doi: 10.1016/j.curtheres.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karmarkar A, Lieberman I. Management of complex regional pain syndrome type II using lidoderm 5% patches. Br J Anaesth. 2007;98:261–262. doi: 10.1093/bja/ael343. [DOI] [PubMed] [Google Scholar]
- 53.Clère F, Delorme-Morin C, George B, et al. 5% lidocaine medicated plaster in elderly patients with postherpetic neuralgia: results of a compassionate use programme in France. Drugs Aging. 2011;28:693–702. doi: 10.2165/11595600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Campbell BJ, Rowbotham M, Davies PS, Jacob P, 3rd, Benowitz NL. Systemic absorption of topical lidocaine in normal volunteers, patients with post-herpetic neuralgia, and patients with acute herpes zoster. J Pharm Sci. 2002;91:1343–1350. doi: 10.1002/jps.10133. [DOI] [PubMed] [Google Scholar]
- 55.Bowsher D. The lifetime occurrence of Herpes zoster and prevalence of post-herpetic neuralgia: a retrospective survey in an elderly population. Eur J Pain. 1999;3:335–342. doi: 10.1053/eujp.1999.0139. [DOI] [PubMed] [Google Scholar]
- 56.Electronic Medicines Compendium. Versatis 5% medicated plaster: summary of product characteristics. 2011. [Accessed July 12, 2012]. Available from: http://www.medicines.org.uk/emc/medicine/19291.
- 57.Maier C, Baron R, Tölle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]