Figure 6. AKT activity is critical for NRF1 nuclear translocation, TFAM expression and mtDNA increase following LPS preconditioning.
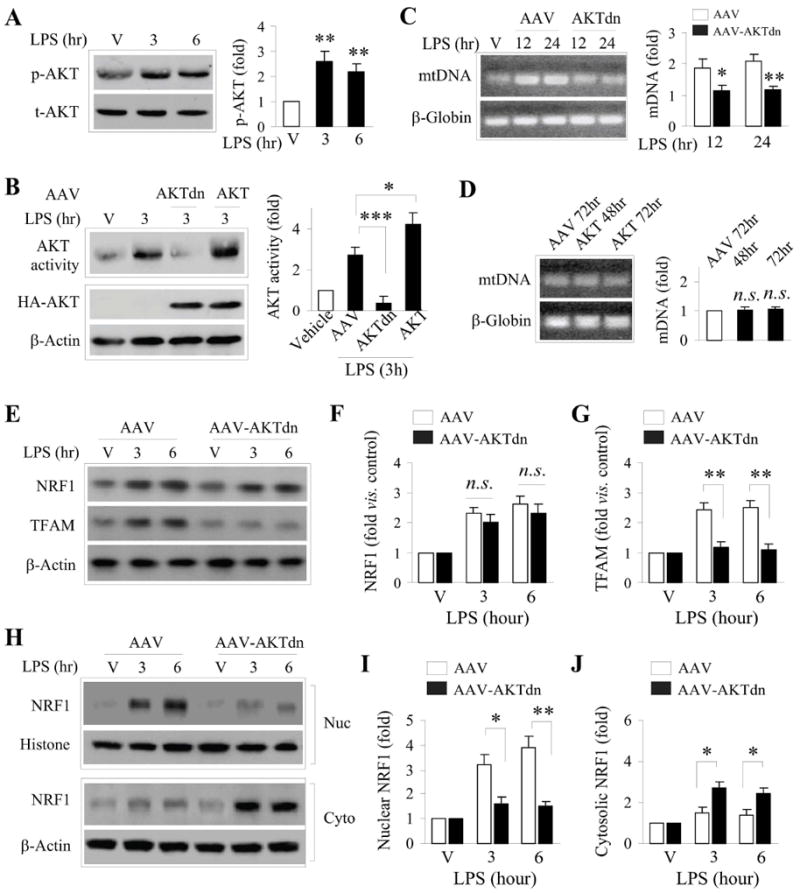
A. LPS preconditioning leads to a rapid increase in Akt phosphorylation (p-AKT) in neurons. Data was from 3 independent cultures, and normalized to total Akt (t-AKT) levels. B. Akt activity, as determined by an in vitro kinase activity assay, was rapidly increased in cell lysates from LPS treated-cultures, and was inhibited by overexpression of the dominant negative AKT (AKTdn). Overexpression of AKT cDNA further increased AKT activity following LPS exposure. C. Overexpression of AKTdn, but not the empty AAV (AAV), suppressed the induction of mtDNA following LPS exposure for 12 or 24 hr. D. Overexpression of AKT alone (without LPS) was insufficient to increase mtDNA content. E-G. Suppression of AKT by AKTdn (but not the empty AAV) overexpression failed to affect NRF1 protein expression (E, F), but significantly reduced TFAM protein expression (E, G) following LPS exposure. H-J. Subcellular fractionation and Western blotting for NFR1 show that inhibition of AKT by AKTdn prevented LPS-induced NRF1 protein levels in the nucleus (H, I) with a concomitant increase of NRF1 protein in the cytosol (H, J). Data were from 3-4 independent cultures, expressed as fold change versus vehicle (non-LPS) treated cultures, and statistically analyzed using ANOVA and post hoc Bonferroni/Dunn tests. *p<0.05, **p<0.01 compared to empty AAV (AAV) or between the indicated groups.
