FIGURE 2:
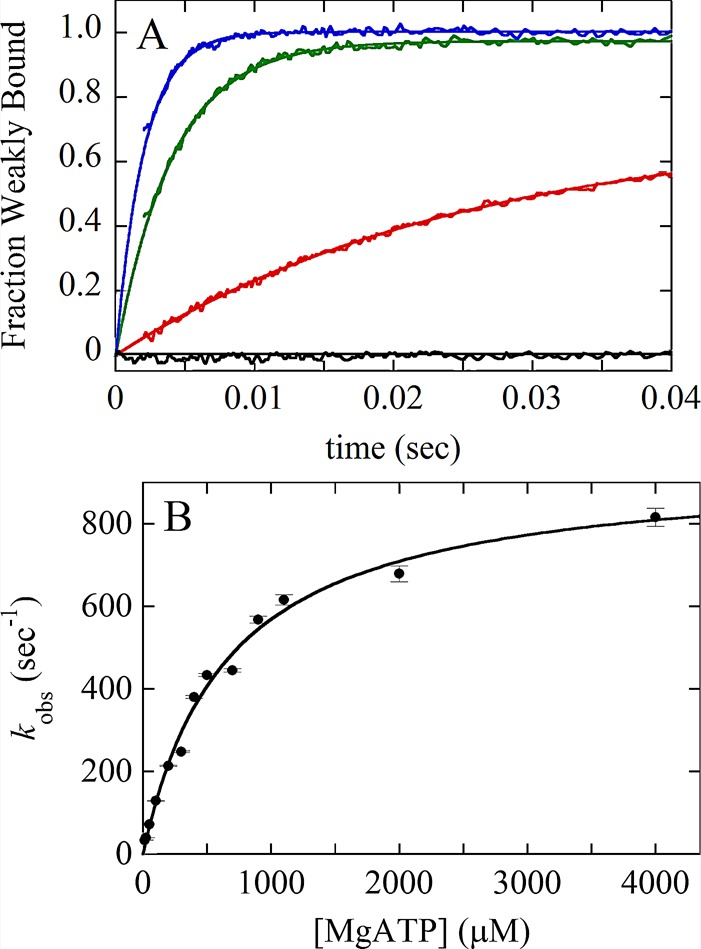
Kinetic analysis of a two-step association reaction: Mg-ATP binding to actomyosin VIIb. (A) Time courses of pyrene fluorescence after mixing 0.1 μM myosin VIIb bound to 0.1 μM pyrene-labeled actin filaments with a range of final concentrations of Mg-ATP: black, 0 μM; red, 25 μM; green, 250 μM; blue, 700 μM. The fluorescence of pyrene-labeled actin filaments is low when associated strongly with myosin without bound nucleotide (AM, black curve). The fluorescence is higher when associated with myosin in a weakly bound conformation induced by bound ATP (AM–ATP*) as observed over time for the other transients. (B) [ATP] dependence of the observed rate constant of fluorescence enhancement. The solid line through the data points is the best fit to a rectangular hyperbola, indicating a multistep mechanism, such as 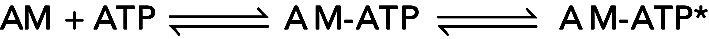 , where * indicates high fluorescence. The first step is rate-limiting at low concentrations of ATP. The second step is rate-limiting at high concentrations of ATP, so the observed rate constant at the plateau gives the sum of the rate constants for the second step, the reversible strong-to-weak actomyosin isomerization reaction. Figure adapted from Henn and De La Cruz (2005).
, where * indicates high fluorescence. The first step is rate-limiting at low concentrations of ATP. The second step is rate-limiting at high concentrations of ATP, so the observed rate constant at the plateau gives the sum of the rate constants for the second step, the reversible strong-to-weak actomyosin isomerization reaction. Figure adapted from Henn and De La Cruz (2005).
