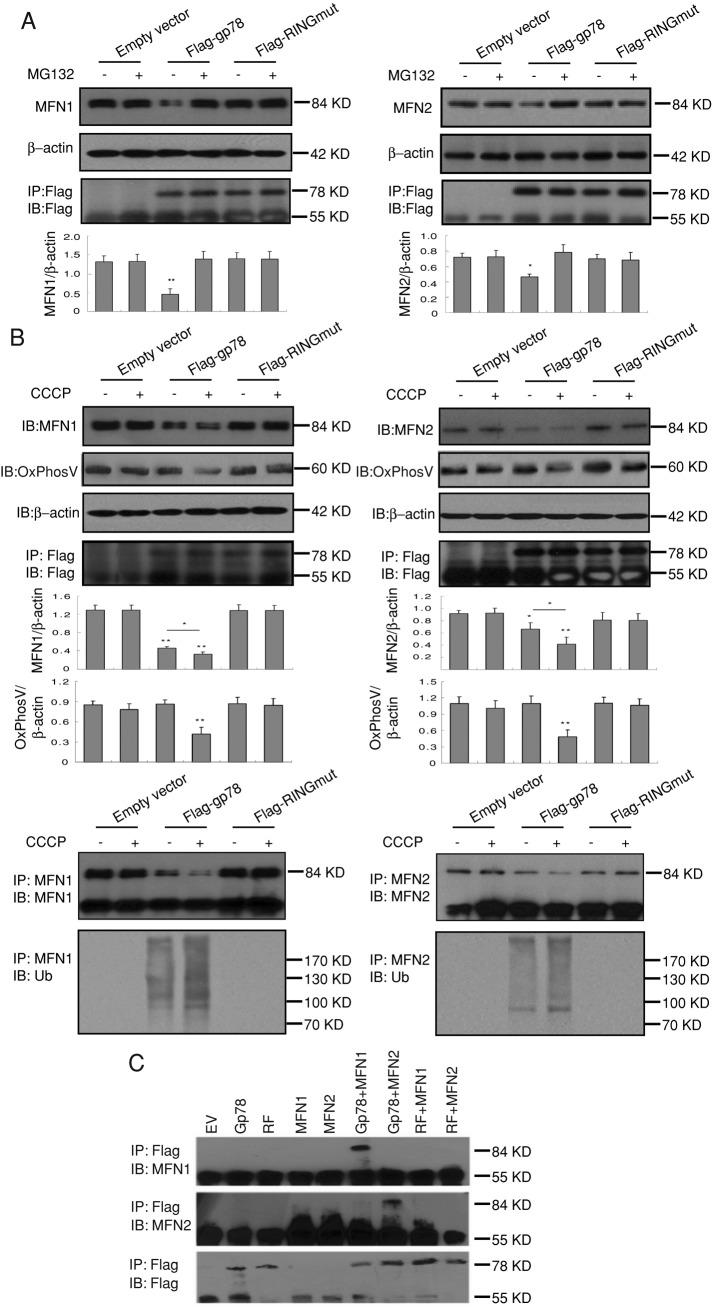
FIGURE 2:
Gp78 induces ubiquitylation and proteasomal degradation of Mfn1 and Mfn2. (A) Endogenous Mfn1 or Mfn2 expression was detected by Western blot in Cos7 cells cotransfected with pcDNA, Flag-gp78, or Flag-RINGmut and treated or not with 30 μM MG132 for 4 h. Flag-gp78 and Flag-RINGmut expression was determined by anti-Flag immunoprecipitation and Western blots. Endogenous Mfn1 and Mfn2 expression was quantified by densitometry relative to β-actin (n = 3, ±SEM; *p < 0.05, **p < 0.01). (B) Endogenous Mfn1, Mfn2, and OxPhosV expression was detected by Western blot in Cos7 cells cotransfected with pcDNA, Flag-gp78, or Flag-RINGmut and treated or not with 20 μM CCCP for 4 h. Immunoprecipitates of endogenous Mfn1 and Mfn2 were immunoblotted with antibodies to Mfn1 or Mfn2, respectively, as well as ubiquitin (Ub; n = 3, ±SEM; *p < 0.05, **p < 0.01). (C) Cos 7 cells were transfected with empty vector (EV), Flag-gp78 (Gp78), Flag-RINGmut (RF), myc-Mfn1 (Mfn1), and myc-Mfn2 alone or cotransfected with Gp78-Mfn1, Gp78-Mfn2, RF-Mfn1, or RF-Mfn2. Anti-Flag immunoprecipitates were probed with antibodies to Mfn1, Mfn2, and Flag. Flag-Gp78 but not Flag-RINGmut interacts with Mfn1 and Mfn2. Flag-gp78 interacts with, reduces expression of, and ubiquitylates Mfn1 and Mfn2, indicating that Gp78 regulates mitofusin levels by targeting these proteins for ERAD.