Figure 5.
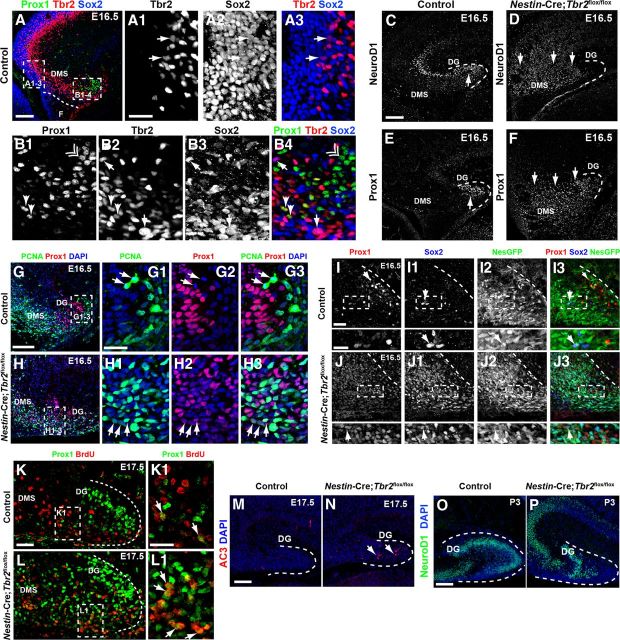
Premature granule cell neurogenesis is apparent in Nestin-Cre;Tbr2flox/flox mice. A–A3, In control mice, Sox2 and Tbr2 are coexpressed in a subset of cells (arrows) in the dentate VZ, whereas Prox1 is not expressed in this region but rather is restricted to the DMS and developing DG (A). However, Sox2+/Tbr2+ cells represent a relatively minor fraction of the total Sox2+ population, consistent with the notion that Sox2 is predominantly expressed in NSCs, whereas Tbr2 is largely restricted to INPs (A1–A3). B1–B4, Within the DMS, Tbr2 and Sox2 continue to be coexpressed in a subset of cells, and these cells are Prox1-negative (arrows). However, coexpression of Tbr2 and Prox1 is apparent in a small subset of Tbr2+ cells, but these Tbr2+/Prox1+ cells are Sox2-negative (arrowheads). Prox1 and Sox2 coexpression is very rare in control animals at E16.5, consistent with downregulation of Sox2 during neuronal differentiation. Accordingly, most Prox1+ cells do not coexpress Sox2 or Tbr2 (double arrowhead). C, In control mice, NeuroD1+ neuroblasts are present in the forming suprapyramidal blade of the DG (arrow) and in the hippocampal CA3 field. Only scattered NeuroD1+ cells are present in the DMS in controls. D, In Nestin-Cre;Tbr2flox/flox mice, numerous NeuroD1+ cells are present in the DMS, which appears to be expanded compared with controls, but relatively few are in the DG itself. E, Prox1 protein is also mostly restricted to the forming DG in control mice, whereas Prox1 is expressed extensively in the expanded DMS of Nestin-Cre;Tbr2flox/flox mice, indicative of an early burst of neurogenesis in mutant animals at E16.5 (F). G–G3, In control mice, Prox1+ neuroblasts rarely proliferate as evidenced by the scarcity of Prox1+/PCNA+ cells in these mice. Arrows illustrate proliferating progenitors (PCNA+) that lack Prox1 expression in a control animal at E16.5 However, in Nestin-Cre;Tbr2flox/flox mice at E16.5, the number of proliferating (PCNA+) Prox1+ neuroblasts is increased (H1–H3, arrows) within the expanded DMS and developing DG. Although PCNA+/Prox1+ cells appear to express Prox1 at low levels, these cells still represent a minority of the total population of Prox1+ neuroblasts in mutant mice (H1–H3). I–I3, In control mice at E16.5, Prox1+ neuroblasts very rarely coexpress the NSC markers Sox2 and Nestin-GFP (NesGFP), consistent with downregulation of these NSC markers in neuroblasts. J–J3, Conversely, in Nestin-Cre;Tbr2flox/flox mice, there is extensive, unusual colocalization of Prox1, Sox2, and Nestin-GFP in the DG. Similar to our observations of Prox1+/PCNA+ cells in mutant mice (H1–H3), Prox1 appears to be expressed at low levels in these mutant Prox1+/Sox2+/NesGFP+ cells (J–J3). K–L1, BrdU pulse-chase labeling (BrdU injected on E15.5. with embryo collection on E17.5) shows that the percentage of Prox1+/BrdU+ cells is increased in Nestin-Cre;Tbr2flox/flox mice (L, L1, arrows) compared with controls (K, K1, arrows), confirming premature neurogenesis in mutant mice during embryonic DG development. M, N, The density of AC3 is increased in Nestin-Cre;Tbr2flox/flox mice, indicating that cell death is increased during this period of premature neurogenesis in mutants (N, arrows). O, P, By P3, the suprapyramidal blade of the DG is easily identifiable in control mice and contains numerous NeuroD1+ neuroblasts, whereas these cells are reduced and misplaced in Nestin-Cre;Tbr2flox/flox mice. Scale bars: A, 100 μm; A1, 50 μm; C, 100 μm; G, 100 μm; G1, 35 μm; I, 35 μm; I (inset), 20 μm; K, 100 μm; K1, 35 μm; M, 75 μm; O, 75 μm. Regions delineated by dashed white boxes are shown in higher magnification in their respective adjacent panels.