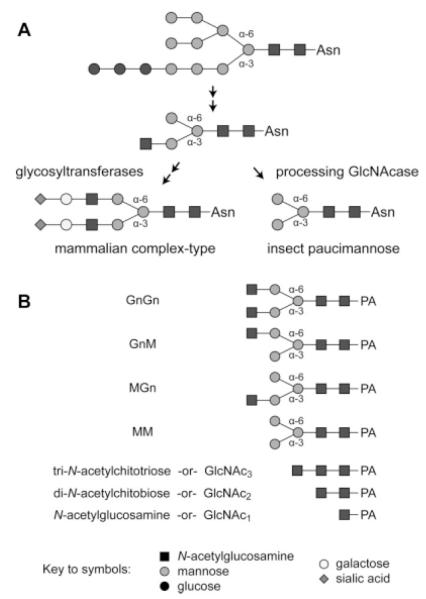
Figure 1. A comparison of insect and mammalian protein N-glycan processing pathways and the structures of the substrates used for β-N-acetylglucosaminidase assays.
(A) The initial steps of N-glycan processing, which are mediated by glucosidase I and II, ER mannosidase I, β-N-acetylglucosaminyltransferase I and Golgi mannosidase II, are common to both cell types and result in the production of a common intermediate with a N-acetylglucosamine residue on the lower, α-3 mannose branch. Mammalian cells express glycosyltransferases, which can add more branches and extend this intermediate to yield terminally sialylated complex N-glycans. Conversely, insect cells express a processing β-N-acetylglucosaminidase that specifically removes the N-acetylglucosamine residue from the α-3 mannose branch to generate the paucimannose structures commonly found on recombinant glycoproteins produced in the BEVS. (B) This panel shows the structures of the glycans used as substrates for the β-N-acetylglucosaminidase assays.