Abstract
BACKGROUND
Although comorbid substance misuse is common in alcohol dependence, and polysubstance abusers (PSU) represent the largest group of individuals seeking treatment for drug abuse today, we know little about potential brain abnormalities in this population. Brain magnetic resonance spectroscopy studies of mono-substance use disorders (e.g., alcohol or cocaine) reveal abnormal levels of cortical metabolites (reflecting neuronal integrity, cell membrane turnover/synthesis, cellular bioenergetics, gliosis) and altered concentrations of glutamate and γ-aminobutyric acid (GABA). The concurrent misuse of several substances may have unique and different effects on brain biology and function compared to any mono-substance misuse.
METHODS
High field brain magnetic resonance spectroscopy at 4 Tesla and neurocognitive testing were performed at one month of abstinence in 40 alcohol dependent individuals (ALC), 28 alcohol dependent PSU and 16 drug-free controls. Absolute metabolite concentrations were calculated in anterior cingulate (ACC), parieto-occipital (POC) and dorsolateral prefrontal cortices (DLPFC).
RESULTS
Compared to ALC, PSU demonstrated significant metabolic abnormalities in the DLPFC and strong trends to lower GABA in the ACC. Metabolite levels in ALC and light drinking controls were statistically equivalent. Within PSU, lower DLPFC GABA levels related to greater cocaine consumption. Several cortical metabolite concentrations were associated with cognitive performance.
CONCLUSIONS
While metabolite concentrations in ALC at one month of abstinence were largely normal, PSU showed persistent and functionally significant metabolic abnormalities, primarily in the DLPFC. Our results point to specific metabolic deficits as biomarkers in polysubstance misuse and as targets for pharmacological and behavioral PSU-specific treatment.
Keywords: Magnetic resonance spectroscopy, substance use comorbidity, alcohol dependence, brain metabolite concentrations, dorsolateral prefrontal cortex, neurocognition
1. INTRODUCTION
Magnetic resonance imaging (MRI) and proton MR spectroscopy (1H MRS) are invaluable tools in addiction research, as they permit non-invasive interrogation of the integrity of multiple aspects of neurobiology. MRS studies that investigate the neurobiological effects of cocaine, amphetamines, or marijuana (i.e., mono-substance dependence) have revealed abnormal levels of markers of neuronal integrity (N-acetylaspartate, NAA), cell membrane turnover/synthesis (choline-containing metabolites, Cho), cellular bioenergetics (Creatine, Cr), astrogliosis (myo-Inositol, mI) and alterations of glutamate (Glu) and γ-aminobutyric acid (GABA), which are the primary excitatory and inhibitory neurotransmitter/neuromodulators in the human brain (for review see (Licata and Renshaw, 2010)). Alterations in brain metabolite concentrations have also been observed in alcohol dependent individuals (ALC), primarily in the frontal lobes (Buhler and Mann, 2011; Durazzo and Meyerhoff, 2007; Mon et al., 2012; Sullivan et al., 2000). We showed recently that concentrations of Glu, NAA and Cr in the anterior cingulate cortex (ACC) of nine-days-abstinent ALC were significantly lower than in healthy controls; mI and γ-aminobutyric acid (GABA) in ACC as well as the other metabolite levels in the dorsolateral prefrontal cortex (DLPFC) and parieto-occipital cortex (POC) were normal (Mon et al., 2012). Over 30 days of abstinence from alcohol, the ACC metabolite concentrations largely normalized (Mon et al., 2012). Furthermore, mono-substance abuse/dependence is associated with neurocognitive dysfunction (Abi-Saab et al., 2005; Di Sclafani et al., 2002; Hester and Garavan, 2004; Lundqvist, 2005; Moeller et al., 2005; Oscar-Berman, 2000; Salo et al., 2002; Simon et al., 2000; Verdejo-Garcia et al., 2011; Volkow et al., 2001), and improvements in functions such as learning, processing speed and working memory have been shown to relate to metabolic changes during abstinence (Meyerhoff et al., 2011).
Alcohol use disorders (AUD) are often accompanied by comorbid misuse of illicit drugs, such as cocaine and methamphetamine (Stinson et al., 2005), and their misuse, individual or combined, is associated with significant neurobiological, neurocognitive and psychiatric abnormalities (Licata and Renshaw, 2010). Individuals with concurrent abuse/dependence on more than one substance including alcohol (i.e., polysubstance abusers or PSU) represent the largest group of individuals seeking treatment for substance use disorders in the United States today (Kedia et al., 2007; Medina et al., 2004). Furthermore, more than 50% of patients treated for drug abuse relapse within one year of treatment (McLellan et al., 2000). Identification of the unique and common neurobiological abnormalities and related neurocognitive characteristics in PSU and ALC will facilitate the development of more efficacious pharmacological and behavioral interventions for these groups. Thus, measuring potentially unique neurobiological abnormalities and related neurocognitive characteristics in treatment seeking PSU and ALC is thought to be of high clinical importance.
Each class of substances alters neuronal integrity and neurotransmission via different mechanisms (Buttner, 2011; Licata and Renshaw, 2010). Their combined abuse may have unique, even different adverse effects on the brain than any mono-substance abuse. While there are numerous neuroimaging studies on effects of individual substances on brain biology, few studies have investigated drug effects in PSU. These can be summarized as follows: two-week-abstinent PSU had prefrontal gray matter (GM) atrophy (Liu et al., 1998). Cocaine use disordered ALC tended to have greater age-related white matter (WM) volume decreases (Bjork et al., 2003) and persistently lower NAA concentrations in the DLPFC than ALC (Meyerhoff et al., 1999). During early withdrawal, PSU demonstrated cerebral phosphorus metabolite alterations (Christensen et al., 1996). Actively using PSU had higher glucose metabolism rates in frontotemporal cortex than drug-free controls (Stapleton et al., 1995); those rates, however, were lower in the right orbitofrontal cortex of one-week-abstinent individuals abusing both methamphetamine and marijuana compared to “pure” methamphetamine abusers (Voytek et al., 2005). Non-abstinent cocaine dependent ALC showed lower frontal GABA levels than controls (Ke et al., 2004). Abstinent ALC with concurrent cocaine dependence had less WM in prefrontal brain regions than those dependent on only one substance (O’Neill et al., 2001). Consistent with these neurobiological abnormalities, studies in PSU have also indicated impaired cognition compared to controls (Di Sclafani et al., 2002; Ersche et al., 2011; Horner, 1997; Selby and Azrin, 1998; Verdejo-Garcia et al., 2004; Verdejo-Garcia et al., 2007). These few reports illustrate that neurobiological correlates of polysubstance use disorders, as well as their associations with neurocognition, are complex and still unclear.
To better understand metabolic alterations in a well-characterized cohort of abstinent PSU, the specific goals of this study were to measure metabolite concentration differences between abstinent PSU and ALC using high-field MRS in brain regions with relevance to the development and maintenance of substance use disorders, and to measure potentially associated neurocognitive (dys)function. Based on the cited literature, we hypothesized unique and functionally significant regional metabolite concentration differences between one-month-abstinent PSU and ALC as well as light drinking controls (LD), and specifically lower cortical NAA (in the DLPFC) and GABA levels in PSU compared to both ALC and LD.
2. MATERIALS AND METHODS
2.1 Participants
All participants provided written informed consent prior to study according to the Declaration of Helsinki and underwent procedures approved by the University of California, San Francisco and the San Francisco VA Medical Center. Twenty eight treatment seeking PSU and 40 ALC were recruited from substance abuse treatment programs of the VA and Kaiser Permanente. All ALC and PSU participants met DSM-IV criteria for alcohol dependence. In addition, PSU participants met DSM-IV criteria for dependence on at least one psychostimulant, with and without marijuana use disorder: cocaine (n=18), methamphetamine (n=4), cocaine and methamphetamine (n=4); 2 PSU were dependent on other substances (opiates, marijuana, and/or ecstasy). Group demographics and relevant substance use characteristics are given in Table 1.
Table 1.
Demographics, laboratory and substance consumption variables for ALC, PSU and LD (mean ± standard deviation). NS: not significant (p>0.05). FTND: Fagerstrom Tolerance Test for Nicotine Dependence, BMI: body mass index, ALT: alanine aminotransferase, AST: aspartate aminotransferase, GGT: γ-glutamyltransferase, WBC: white blood cell counts, RBC: red blood cell counts.
| Variable | LD | ALC | PSU | p (LD-ALC) |
p (LD-PSU) |
p (ALC-PSU) |
|---|---|---|---|---|---|---|
| n (male, female) | 16 (15, 1) | 40 (37, 3) | 28 (26,2) | - | - | - |
| Age [years] | 49.0 ± 10.1 | 52.1 ± 9.1 | 45.3 ± 9.6 | NS | NS | 0.004 |
| Education [years] | 15.4 ± 2.7 | 13.6 ± 1.8 | 12.8 ± 1.4 | 0.001 | 0.000 | NS |
| Smoker n | 6 | 26 | 18 | - | - | - |
| NART | 116.4 ± 8.0 | 114.8 ± 8.3 | 106.4 ± 9.2 | NS | 0.001 | 0.000 |
|
onset heavy drinking
[age] |
- | 25.0 ± 9.1 | 20.7 ± 7.8 | - | - | NS |
| Months heavy drinking | - | 269 ± 119 | 238 ± 132 | - | - | NS |
| Sober days (alcohol) | - | 32.7 ± 7.5 | 28.6 ± 11.8 | - | - | NS |
| Sober days (any drug) | - | 32.7 ± 7.5 | 27.0 ± 11.9 | - | - | 0.021 |
|
1 year avg. (alcohol)
[Drinks/month] |
22 ± 24 | 377 ± 258 | 302 ± 326 | 0.000 | 0.001 | NS |
|
3 year avg. (alcohol)
[Drinks/month] |
22 ± 24 | 340 ± 206 | 300 ± 318 | 0.000 | 0.000 | NS |
|
Life time avg. (alcohol)
[Drinks/month] |
23 ± 17 | 211 ± 109 | 261 ± 261 | 0.000 | 0.000 | NS |
|
1 year avg. (cocaine)
[g/month] |
- | - | 82.2± 106.2 | - | - | - |
|
Life time avg. (cocaine)
[g/month] |
- | - | 75.2 ± 96.2 | - | - | - |
|
1 year avg.
(methamphetamine) [g/month] |
- | - | 21.2 ± 29.1 | - | - | - |
|
Life time avg.
(methamphetamine) [g/month] |
- | - | 14.1 ± 13.6 | - | - | - |
| FTND total (smokers) | 5.7 ± 2.4 | 4.7 ± 1.7 | 4.0 ± 1.6 | NS | 0.049 | NS |
| cigarettes/day | 21.2 ± 9.7 | 16.8 ± 7.9 | 10.0 ± 7.3 | NS | 0.004 | 0.005 |
| BMI | 25.5± 1.1 | 26.3± 0.7 | 27.9± 0.9 | NS | 0.085 | NS |
| Prealbumin [mg/dl] | 29.2 ± 5.8 | 25.8 ± 5.7 | 30.2 ± 7.0 | NS | NS | 0.009 |
| GGT [U/l] | 42.9 ± 78.5 | 72.1 ± 117.4 | 39.9 ± 71.1 | NS | NS | NS |
| Albumin [mg/dl] | 4.2 ± 0.3 | 4.1 ± 0.3 | 4.2 ± 0.2 | NS | NS | NS |
| AST [U/l] | 49.2 ± 82.1 | 41.2 ± 52.4 | 39.9 ± 60.9 | NS | NS | NS |
| ALT [U/l] | 51.0 ± 93.2 | 44.2 ± 67.6 | 41.0 ± 51.1 | NS | NS | NS |
| WBC [K/cmm] | 6.1 ± 1.6 | 7.2 ± 2.1 | 6.2 ± 1.7 | 0.10 | NS | 0.039 |
| RBC [M/cmm] | 4.7 ± 0.4 | 4.5 ± 0.5 | 4.6 ± 0.4 | NS | NS | NS |
| Hemoglobin [g/dl] | 15.0 ± 1.0 | 14.4 ± 1.3 | 14.4 ± 1.0 | NS | NS | NS |
| Hematocrit [%] | 43.3 ± 3.3 | 42.0 ± 3.9 | 42.6 ± 3.1 | NS | NS | NS |
| MCV [fl] | 92.0 ± 4.1 | 93.7 ± 4.5 | 91.8 ± 4.9 | NS | NS | NS |
| BDI | 5.8 ± 2.2 | 12.2 ± 1.4 | 11.7 ± 1.5 | 0.017 | 0.031 | NS |
| State t anxiety y2 | 35.0 ± 3.0 | 44.3 ± 1.8 | 43.0 ± 2.0 | 0.010 | 0.030 | NS |
| State t anxiety y1 | 26.3 ± 2.8 | 33.9 ± 1.6 | 34.2 ± 1.8 | 0.020 | 0.019 | NS |
At study date, ALC and PSU were abstinent from alcohol and other substances, except nicotine, for approximately one month. Further inclusion and exclusion criteria are fully detailed elsewhere (Durazzo et al., 2004). Participants were excluded for neurological or psychiatric disorders known to affect neurobiology or neurocognition. Hepatitis C, type-2 diabetes, hypertension, and unipolar mood disorders were permitted given their high prevalence in substance use disorders (Hasin et al., 2007; Mertens et al., 2003; Mertens et al., 2005; Parekh and Klag, 2001; Stinson et al., 2005). Sixteen light drinking controls (LD) without history of biomedical and/or psychiatric conditions known to influence the measures obtained in this study were recruited from the local community. Data from 85% of ALC and 94% of LD participants contributed to previous analyses (Mon et al., 2012).
2.2 Clinical Assessment
ALC and PSU participants completed the Structured Clinical Interview for DSM-IV Axis I Disorder Patient Edition, Version 2.0 (First et al., 1998), and LD participants were administered the accompanying screening module. Within one day of the MR study, all participants filled out questionnaires that assessed depression (Beck Depression Inventory; Beck, 1978) and anxiety symptomatologies (State-Trait Anxiety Inventory, Y-2; Spielberger et al., 1977). Alcohol consumption was assessed with the lifetime drinking history semi-structured interview (Skinner and Sheu, 1982; Sobell and Sobell, 1990; Sobell et al., 1988), which yielded estimates of the average number of alcoholic drinks consumed per month over 1 year and 3 years, before enrollment and over lifetime. For PSU, lifetime substance use history (other than alcohol) was assessed with an in-house interview questionnaire based on the Addiction Severity Index (McLellan et al., 1992), NIDA Addictive Drug Survey (Smith, 1991), drinking history, and Axis I disorders Patient Edition, Version 2.0 (SCID-I/P; First et al., 1998). This instrument gathers information relevant to phases of drug use for each substance a participant has a current or past disorder diagnosis on; including age of first and last use, number of total lifetime phases, duration of individual and total lifetime phases (including phases of abstinence), frequency and quantity of use during each phase, and route of administration. It includes conversion of money spent per day to one metric, using catchment area-specific conversion norms. Thus, monthly averages for grams of cocaine and/or methamphetamine over 1 year prior to enrolment and over lifetime were estimated. Level of nicotine dependence was assessed via the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991), and total numbers of years of smoking and average daily cigarettes currently smoked were recorded. To evaluate basic nutritional and erythrocyte status and hepatocellular injury, we obtained laboratory tests for serum albumin, pre-albumin, alanine aminotransferase, aspartate aminotransferase and γ-glutamyltransferase, white and red blood cell counts, hemoglobin, and hematocrit.
2.3 Neurocognitive Assessment
Within 3 days of the MR study, all participants completed a neurocognitive battery focusing on working memory (Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span), processing speed (WAIS-III Digit Symbol Coding, Symbol Search; Wechsler, 1997), visuospatial learning and memory (Brief Visual memory Test-Revised, BVMT-R; Benedict, 1997), as well as auditory-verbal learning and memory (California Verbal Learning Test-II, CVLT-II: immediate recall trials 1-5 (learning), average of short and long delay free recall, and average of short and long delay cued recall (memory); Delis et al., 2000). (For details see Durazzo et al., 2007.) Premorbid verbal intelligence was assessed using the American National Adult Reading Test (NART; Grober and Sliwinski, 1991). Group comparisons on these measures will be detailed in a separate report.
2.4 MR Acquisition and Processing
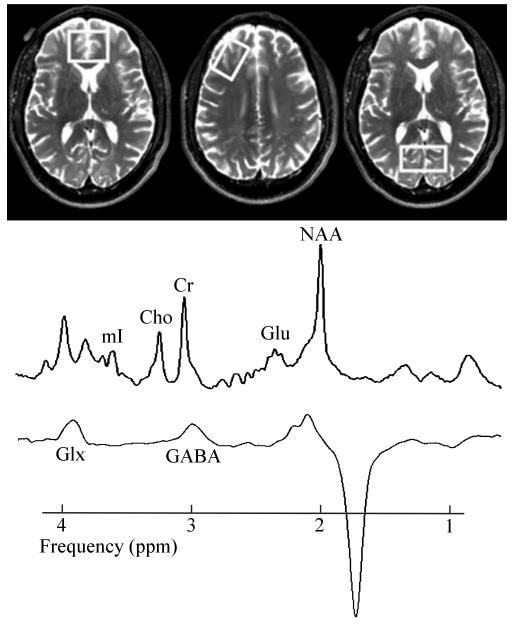
MR data were acquired on a 4 Tesla Bruker MedSpec system with a Siemens Trio console (Siemens, Erlangen, Germany) using an 8-channel transmit-receive head coil. 3D sagittal T1-weighted and 2D axial T2-weighted images were acquired using Magnetization Prepared Rapid Gradient imaging (1×1×1 mm3 resolution) and turbo spin-echo (0.9×0.9×3 mm3 resolution) sequences respectively. Volumes-of-interest (VOIs) for MRS were placed over the ACC (35×25×20 mm3), POC (20×40×20 mm3) and right DLPFC (40×20×20 mm3) (Figure 1), maximizing GM content as displayed on the structural MR images. NAA, Cr, Cho, mI and Glu signals were acquired with a Stimulated Echo Acquisition Mode (STEAM) sequence (Frahm et al., 1987). GABA signal was acquired from the same VOIs with a modified J-editing sequence (MEGA PRESS; Kaiser et al., 2008). MR images were segmented into GM, WM and cerebrospinal fluid (CSF; Van Leemput et al., 1999) to estimate tissue fraction and CSF contributions to each VOI. (A full description of the spectral processing methods can be found in Mon et al., 2012.) ALC and LD were scanned fully contemporaneously, while PSU were scanned contemporaneously and in random order with the latter half of the cohort. Data acquisition and processing conditions were the same for all participants, and data processing was done by operators blind to participant diagnosis.
Figure 1.
Representative VOI placements (left: ACC, center: DLPFC, right: POC), STEAM and J-edited GABA spectra obtained from PSU (note: intensity scale differs between spectra). Spectra shown as fitted (after DC-correction and apodization). Glx: glutamate + glutamine.
2.5 Statistical Analyses
Separate univariate analyses of covariance (ANCOVA) were performed for each VOI (DLPFC, ACC, and POC) and each metabolite, followed up with planned pairwise comparisons to test for group differences among PSU, ALC and LD in metabolite concentrations using SPSS, v20. Since age (e.g., Schuff et al., 2001) and differences in brain tissue contributions to the VOIs (GM, WM, CSF; Jansen et al., 2006) affect observed brain metabolite levels, we covaried for age and GM-tissue contribution, the target of our VOI placement. The number of acquired J-edited (GABA) spectra was often lower than for STEAM spectra (DLPFC: PSU=20 vs. 27, ALC=24 vs. 30, LD=13 in both; ACC: PSU=21 vs. 20, ALC=27 vs. 37, LD=14 in both; POC: PSU=20 vs. 23, ALC=30 vs. 35, LD=9 vs. 12). ANCOVA was also used to test for differences in participants’ characterization measures. In pairwise group comparisons of metabolite levels, we corrected alpha levels (0.05) to account for the multiplicity of metabolites in each VOI via a modified Bonferroni procedure (Sankoh et al., 1997). This approach yields adjusted alpha levels for each VOI separately using the number of metabolites under investigation (six) and their average inter-correlation coefficients (DLPFC: r=.54, ACC: r=.39, POC: r=.53); corresponding adjusted alpha levels for pairwise group comparisons were 0.022 (DLPFC), 0.017 (ACC) and 0.020 (POC). Effect sizes were calculated via Cohen’s d (Cohen, 1988). For each VOI within PSU, correlations (Spearman’s Rho) between metabolite concentrations and neurocognitive measures (raw scores), drug consumption variables, age and days of abstinence were calculated. Where necessary, relationships between metabolite levels and other measures were corrected for age (partial correlations).
3. RESULTS
3.1 Participants Characterization
Characteristics for ALC, PSU and LD are shown in Table 1. PSU were younger than ALC and had lower NART scores than ALC or LD. PSU and ALC had fewer years of education than LD. Although PSU had a higher prealbumin concentration than ALC and LD, and ALC had higher white blood cell counts than PSU and LD, these and other clinical laboratory measures were within the normal range for all groups. PSU and ALC did not differ on any alcohol drinking measure. The number of days abstinent from alcohol at time of study (sober days) was equivalent at about 30 days; PSU were abstinent from any drug approximately 6 fewer days than from alcohol. PSU and ALC were equivalent on measures of depressive and anxiety symptomatologies, both had higher measures than LD. Among smokers, the Fagerstroem test indicated moderate nicotine dependence in PSU and moderate to high dependence in ALC and LD; PSU smoked significantly fewer cigarettes per day than ALC and LD. The ALC and PSU group each contained four participants diagnosed with hepatitis C. Within each group, however, outcome measures of those with and without hepatitis C were equivalent. The same applied to participants with (10 ALC, 5 PSU) and without controlled hypertension. Finally, total brain tissue, GM, WM and CSF fractions in the three VOIs did not differ significantly between groups (mean values: DLPFC: GM=0.38, WM=0.55, CSF=0.06; ACC: GM=0.46, WM=0.33, CSF=0.20; POC: GM=0.61, WM=0.29, CSF=0.09; unassigned 1%), and omitting GM-tissue contribution as covariate did not appreciably change the results of observed group differences for any of the metabolites and VOIs
3.2 Group Comparison of Metabolite Concentrations
Univariate tests were significant for group differences in the DLPFC: NAA (p=0.004), Cr (p=0.015), Cho (p=0.020) and mI (0.018). Table 2 shows mean metabolite concentrations, and pairwise group statistics. In planned pairwise comparisons of metabolite levels in the DLPFC, PSU had significantly lower NAA, Cho and mI concentrations than both ALC and LD (p ≤ .022). Cr was significantly lower in PSU compared to ALC and tended to be lower compared to LD. Effect sizes for differences between PSU and ALC were moderate for Cho, Cr, mI and strong for NAA. Similar patterns were observed between PSU and LD. In the ACC, no significant group differences were found. However, PSU showed a strong trend to lower GABA concentrations compared to ALC and LD (moderate effect sizes: 0.64<ES<0.73). Effect sizes for all ACC metabolites but mI in comparisons of PSU to LD were moderate to strong. In the POC, ALC showed a trend to elevated Cho levels compared to both PSU and LD, whereas Cho was similar in PSU and LD. The statistically weaker differences between PSU/ALC and LD were likely related to the smaller LD sample.
Table 2.
DLPFC, ACC and POC metabolite concentrations (mean ± standard deviation) in institutional units [i.u.]. NS: Not significant; p-values that are significant after Bonferroni adjustment are bolded. Bonferroni adjusted alpha levels: DLPFC: p=0.022, ACC: p=0.017, POC: p=0.020.
| Region | Metabolite | p-value | Metabolite concentration [i.u.] | ||||
|---|---|---|---|---|---|---|---|
| LD-ALC | LD-PSU | ALC-PSU | LD | ALC | PSU | ||
| DLPFC | NAA | NS | 0.018 | 0.002 | 5.10 ± 0.84 | 5.12 ± 0.84 | 4.41 ± 0.84 |
| Cr | NS | 0.053 | 0.006 | 4.28 ± 0.71 | 4.34± 0.70 | 3.80 ± 0.72 | |
| Cho | NS | 0.056 | 0.008 | 1.06 ± 0.17 | 1.07 ± 0.17 | 0.95 ± 0.17 | |
| Glu | NS | NS | NS | 3.06 ± 0.69 | 2.90 ± 0.69 | 2.80 ± 0.69 | |
| mI | NS | 0.029 | 0.010 | 3.60 ± 0.81 | 3.56 ± 0.81 | 2.99 ± 0.81 | |
| GABA | NS | NS | NS | 2.10 ± 0.61 | 2.09 ± 0.61 | 1.86 ± 0.61 | |
| ACC | NAA | NS | NS | NS | 5.49 ± 1.02 | 4.92 ± 1.02 | 4.96 ± 1.02 |
| Cr | NS | NS | NS | 4.60 ± 0.97 | 3.99 ± 0.97 | 4.05 ± 0.97 | |
| Cho | NS | NS | NS | 1.31 ± 0.33 | 1.25 ± 0.33 | 1.17 ± 0.34 | |
| Glu | NS | NS | NS | 4.03 ± 0.94 | 3.73 ± 0.95 | 3.44 ± 0.99 | |
| mI | NS | NS | NS | 4.24 ± 1.01 | 4.15 ± 1.02 | 4.28 ± 1.04 | |
| GABA | NS | 0.095 | 0.028 | 1.32 ± 0.39 | 1.36 ± 0.40 | 1.09 ± 0.35 | |
| POC | NAA | NS | NS | NS | 5.11 ± 0.88 | 5.44 ± 0.88 | 5.10 ± 0.88 |
| Cr | NS | NS | NS | 4.24 ± 0.73 | 4.50 ± 0.74 | 4.27 ± 0.75 | |
| Cho | 0.058 | NS | 0.029 | 0.73 ± 0.19 | 0.85 ± 0.20 | 0.74 ± 0.19 | |
| Glu | NS | NS | NS | 3.89 ± 0.72 | 4.02 ± 0.72 | 3.74 ± 0.72 | |
| mI | NS | NS | NS | 3.30 ± 0.74 | 3.25 ± 0.74 | 3.06 ± 0.74 | |
| GABA | NS | NS | NS | 1.42 ± 0.47 | 1.53 ± 0.47 | 1.47 ± 0.47 | |
Considering that cigarette smoking affected brain metabolite concentrations in individuals with AUD (Durazzo et al., 2004, 2006), in secondary analysis we covaried for smoking status (non-smoker and smoker) in the three-group-comparisons. This did not significantly alter the aforementioned results. Similarly, controlling for years of education, days of sobriety, body mass index, NART, depression, anxiety and drinking severity measures did not alter the above group differences. Therefore, those variables were not used as covariates in our final analyses.
3.3 Correlations among main outcome measures within PSU
3.3.1 Metabolite concentrations and neurocognition
Metabolite concentrations and cognitive performance are typically robustly associated with age. In the combined cohort, age-metabolite relationships were found in DLPFC and POC, primarily with Cho and Cr; in PSU only, DLPFC mI was significantly related to age (p=0.017, r=0.46), whereas statistical trends for age-metabolite relationships were seen in ALC and LD only. Therefore, we controlled for the effects of age when correlating other outcome measures. in the DLPFC, NAA was positively related to visuospatial and working memory raw scores (see Table 3), in the ACC, NAA and Cho were positively related to visuospatial learning and processing speed raw scores of the BVMT-R and WAIS-III, respectively. GABA in the ACC showed an inverse relationship with auditory-verbal learning (CVLT-II raw scores), whereas in the POC, GABA was positively correlated with the visuospatial memory raw score of the BVMT-R. Different associations were observed in ALC, who showed inverse relationships between DLPFC mI and processing speed and between POC mI and visuospatial learning (both: p<0.014, r>-0.48). No significant metabolite level-function relationships were found in LD.
Table 3.
Age corrected partial correlations (r*) between brain metabolite concentrations and neurocognitive measures within PSU.
| Region | Metabolite concentration and cognitive measure |
r* | p |
|---|---|---|---|
| DLPFC | NAA and visuospatial memory NAA and working memory |
0.42 0.53 |
0.045 0.019 |
| ACC | NAA and visuospatial learning Cho and processing speed GABA and auditory-verbal learning |
0.51 0.55 -0.48 |
0.039 0.023 0.049 |
| POC | GABA and visuospatial memory | 0.51 | 0.031 |
3.3.2 Metabolite levels and substance consumption
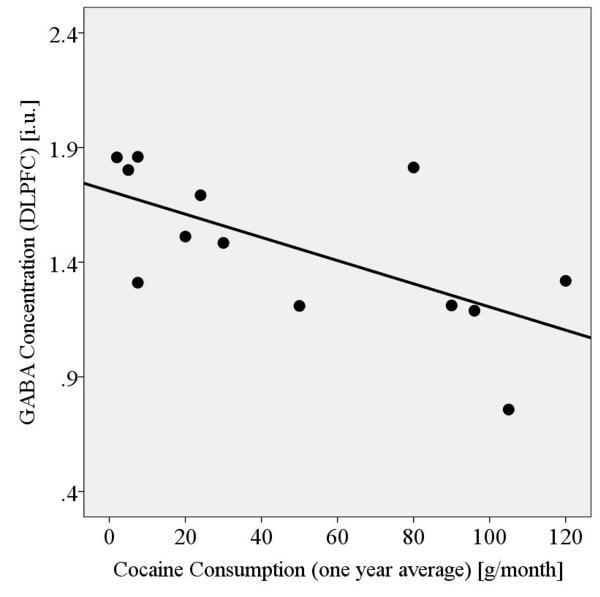
Within 13 PSU participants, lower DLPFC GABA was related to greater monthly cocaine consumption measures, averaged over both life time (p<0.019, r=-.64) and one year (p<0.016, r=-.65) (Figure 2), and independent of average life time alcoholic drinks/month. Methamphetamine consumption in eight PSU was not related to any metabolite concentrations in any VOI. Moreover, in PSU, which showed significantly lower mI than ALC and LD, more days of abstinence from any drug were related to higher DLPFC mI levels (p=0.01, r=0.50).
Figure 2.
DLPFC GABA concentrations of cocaine dependent PSU in relation to their monthly cocaine consumption (averaged over last year before abstinence).
4. DISCUSSION
We employed high-field 1H MRS and neuropsychological assessments to compare regional brain metabolite levels in the ACC, DLPFC and POC and examine their functional relevance in alcohol dependent individuals with and without comorbid illicit substance dependence after one month of abstinence. The ACC and DLPFC are important components of the brain reward/executive oversight system (BREOS), a network that is critically involved in the development and maintenance of all forms of addictive disorders (Goldstein et al., 2009; Volkow et al., 2011).
In the DLPFC, PSU demonstrated significantly lower NAA, Cho, mI and Cr concentrations than ALC. DLPFC GABA levels were inversely related to cocaine use. In the ACC, GABA concentrations tended to be lower in PSU compared to both ALC and LD. Moreover, cortical metabolite levels were significantly related to neurocognitive measures, affirming the functional relevance of absolute MRS measures in PSU. Finally, metabolite levels in ALC and LD were statistically equivalent, presumably due to longitudinal recovery of metabolite levels found low in ALC earlier in sobriety (Mon et al., 2012).
Our results suggest that PSU, when compared to both LD and ALC, had abnormal neuronal integrity (lower NAA), abnormalities in glial or cell membrane synthesis/turnover (lower Cho), altered glial content or osmoregulation (lower mI), and trends to abnormal cellular bioenergetics (lower Cr; Licata and Renshaw, 2010). These abnormalities at one month of abstinence were specific to the DLPFC, a region critical for such integrating activities as planning and organization, response inhibition, working memory, reasoning, problem solving and set shifting (Goldstein and Volkow, 2011; Petrides, 2005). There were strong trends to GABAergic abnormalities in the ACC, a region critical to decision making and response inhibition. Since the detected metabolic differences were shown to be unrelated to age, alcohol consumption, body mass index, smoking status, depression and anxiety symptomatologies, the misuse of illicit drugs in PSU is likely related to the observed metabolite group differences. Considering research in both ALC (reviewed in Licata and Renshaw, 2010; Meyerhoff et al., 2011) and cocaine dependent ALC (Meyerhoff et al., 1999), we further conclude that neurobiological abnormalities in alcohol dependent individuals with comorbid illicit substance dependence are qualitatively and regionally different at one month of abstinence and longer lasting than those related to alcohol dependence alone.
Although not statistically significant, we observed differences of moderate effect sizes in ACC metabolite levels between PSU and LD (e.g., NAA, Glu, GABA). This may indicate greater metabolic abnormalities in the ACC of PSU earlier in sobriety, similar to what is observed in one-week abstinent ALC (Mon et al., 2012) and opiate-depended individuals (Yucel et al., 2007). This data therefore suggests partial metabolic recovery in PSU with extended abstinence, reminiscent of ALC (Durazzo et al., 2006; Mon et al., 2012). Longitudinal metabolic changes in abstinent PSU are also suggested by the association of DLPFC mI with days of abstinence.
4.1 Myo-Inositol
Other reports showed lower NAA, Cho and Cr in anterior brain regions associated with mono-substance use disorders (reviewed in Licata and Renshaw, 2010). However, whereas most MRS studies on mono-substance dependence reported higher mI in frontal brain, we found lower mI concentrations in the DLPFC of PSU compared to both ALC and LD. Although the precise function(s) of cerebral mI is still debated (Griffin et al., 2002; Moore et al., 1999), it is suggested to be a marker of astroglial content, with elevations mostly interpreted as astroglial hypertrophy and/or proliferation adversely affecting brain function (Licata and Renshaw, 2010; Meyerhoff et al., 2011; Mon et al., 2012; Schweinsburg et al., 2001). Since the DLPFC VOI in this study contains a majority of WM tissue, lower mI may indicate glial content (e.g., astrocytes) loss and/or damage related to polydrug consumption. Reductions in mI could also occur through inhibition of its synthesis or uptake (Coupland et al., 2005). The interaction of two or more different drugs used concurrently leads to formation of adducts of the primary compounds and their metabolites (e.g., cocaethylene, benzoylecgonine, norcocaine, norcocaethylene (Cardona et al., 2006; Toennes et al., 2003)). They may have additional toxic and/or inflammatory effects (Farooq et al., 2009) and/or might also inhibit mI synthesis or uptake.
4.2 GABA
The trends to low GABA concentration in the ACC of PSU (17-20% lower than in LD or ALC) indicate that the GABA system is altered in PSU. Our GABA findings are consistent with Ke et al. (2004), who showed low GABA levels in the prefrontal cortex of a cohort concurrently dependent on alcohol and cocaine. It is therefore likely that our GABA findings were driven by the 18 PSU individuals dependent on these two substances. However, their ACC GABA levels were equivalent to those in the 10 PSU individuals dependent on substances other than cocaine. This, together with our finding of largely normal GABA levels in ALC after both one week and one month of abstinence (Mon et al., 2012), suggest that GABA abnormalities in the ACC are associated with concurrent use of alcohol and illicit drugs in PSU, but not with alcohol dependence alone.
Our interpretation of lower metabolite levels (GABA, NAA, Cr, Cho, mI) relating to cell injury or dysfunction associated with polydrug use is further supported by three observations: First, we observed worse neurocognitive performance of PSU compared to ALC and LD (results will be published elsewhere), and neurocognitive deficits have been associated frequently with metabolic abnormalities (Meyerhoff and Durazzo, 2008). Secondly, the positive correlation in PSU between days of abstinence and DLPFC mI levels suggests an increase of mI into the normal range (some degree of metabolic recovery) during the first 10-45 days of abstinence. Lastly, the inverse relationship between DLPFC GABA levels and cocaine consumption in PSU, together with lower ACC GABA in PSU vs. LD and ALC, suggest that the prefrontal GABA metabolic pool is impacted by chronic cocaine consumption. On these grounds, we share the view put forth by Johnson and colleagues, who suggest that efforts, which increase GABA levels/activity in PSU (e.g., topiramate treatment), may offer fruitful treatment possibilities (Johnson, 2005). However, our observation of a simultaneous decrease of four different metabolites (incl. mI) in the DLPFC of PSU might also be explained by possible substance use associated neuronal/glial edema, leading to higher water, thus lower overall metabolite concentrations. We suggest that both cell injury and/or swelling (in GM and/or WM) might occur in the DLPFC of PSU.
4.3 Study Limitations and Outlook
Given our PSU cohort was abstinent for about one month, greater or different metabolic abnormalities may have been present earlier in abstinence, as observed in the ACC-specific metabolite abnormalities in one-week-abstinent ALC (Mon et al., 2012). We suggest metabolic recovery occurring in PSU. Longitudinal follow-up will assist in clarifying if the observed abnormalities are reversible, or if our observations are influenced by premorbid and/or comorbid factors not assessed in this study. Few metabolite levels correlated with substance and alcohol consumption quantities, and notwithstanding the known limitations of self-report, this suggests that metabolite level abnormalities may be (in part) premorbid. If this were the case, brain metabolite concentrations could serve as biomarkers or risk factors for the development of PSUD. Larger study samples will help identify the degree to which comorbid factors such as cigarette smoking may have influenced our findings. Another observation in need for further investigation is the age-related DLPFC mI increase in PSU, not observed in ALC or LD: polydrug consumption may be associated with alterations of the brain ageing process, similar to what was shown in cocaine dependent individuals (Ersche et al., 2012). Furthermore, neurobiological abnormalities in the ACC and DLPFC are known to be associated with increased risk of relapse to substance use (Baler and Volkow, 2006; Goldstein et al., 2009; Heatherton and Wagner, 2011; Volkow et al., 2011). Hence, it is also critical to investigate if the abnormal regional brain metabolite levels detected in this study are related to relapse risk, as observed in those with AUD (Durazzo et al., 2008, 2010). Different relapse rates in PSU and ALC may be related to their unique neurobiological and cognitive differences demanding differently designed treatment approaches for these disorders.
4.4 Conclusions
MRS-derived metabolite concentrations show that PSU are uniquely different from ALC at one month of abstinence. PSU showed persistent metabolic abnormalities, primarily in the DLPFC, whereas metabolite levels in ALC are normal, after having recovered from abnormal levels in the ACC (Mon et al., 2012). These abnormalities reflect neuronal and glial injury/dysfunction and some are also related to neurocognition. Our results point to specific metabolic abnormalities as polydrug abuse biomarkers and as potential targets for pharmacological and behavioral PSU-specific treatment aimed at decreasing high relapse rates in PSU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abi-Saab D, Beauvais J, Mehm J, Brody M, Gottschalk C, Kosten TR. The effect of alcohol on the neuropsychological functioning of recently abstinent cocaine-dependent subjects. Am. J. Addict. 2005;14:166–178. doi: 10.1080/10550490590924854. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol. Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test - Revised: Professional Manual. Psychological Assessment Resources, Inc.; Odessa, FL: 1997. [Google Scholar]
- Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. Am. J. Psychiatry. 2003;160:2038–2045. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol. Clin. Exp. Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Buttner A. Review: the neuropathology of drug abuse. Neuropathol. Appl. Neurobiol. 2011;37:118–134. doi: 10.1111/j.1365-2990.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- Cardona PS, Chaturvedi AK, Soper JW, Canfield DV. Simultaneous analyses of cocaine, cocaethylene, and their possible metabolic and pyrolytic products. Forensic Sci. Int. 2006;157:46–56. doi: 10.1016/j.forsciint.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Christensen JD, Kaufman MJ, Levin JM, Mendelson JH, Holman BL, Cohen BM, Renshaw PF. Abnormal cerebral metabolism in polydrug abusers during early withdrawal: a 31P MR spectroscopy study. Magn. Reson. Med. 1996;35:658–663. doi: 10.1002/mrm.1910350506. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Coupland NJ, Ogilvie CJ, Hegadoren KM, Seres P, Hanstock CC, Allen PS. Decreased prefrontal myo-inositol in major depressive disorder. Biol. Psychiatry. 2005;57:1526–1534. doi: 10.1016/j.biopsych.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd Edition The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol. Clin. Exp. Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol. Clin. Exp. Res. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol Alcohol. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front. Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J. Stud. Alcohol Drugs. 2010;71:278–289. doi: 10.15288/jsad.2010.71.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol. Clin. Exp. Res. 2007;31:1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Mol. Psychiatry. 2012 doi: 10.1038/mp.2012.31. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–765. [PubMed] [Google Scholar]
- Farooq MU, Bhatt A, Patel M. Neurotoxic and cardiotoxic effects of cocaine and ethanol. J. Med. Toxicol. 2009;5:134–138. doi: 10.1007/BF03161224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision) Biometrics Research Department; New York, NY: 1998. [Google Scholar]
- Frahm J, Merboldt KD, H„nicke W. Localized proton spectroscopy using stimulated echoes. J. Magn. Reson. 1987;72:502–508. doi: 10.1002/mrm.1910170113. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn. Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JL, Bollard M, Nicholson JK, Bhakoo K. Spectral profiles of cultured neuronal and glial cells derived from HRMAS (1)H NMR spectroscopy. NMR Biomed. 2002;15:375–384. doi: 10.1002/nbm.792. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J. Clin. Exp. Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MD. Cognitive functioning in alcoholic patients with and without cocaine dependence. Arch. Clin. Neuropsychol. 1997;12:667–676. [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kedia S, Sell MA, Relyea G. Mono- versus polydrug abuse patterns among publicly funded clients. Subst. Abuse Treat. Prev. Policy. 2007;2:33. doi: 10.1186/1747-597X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann. N. Y. Acad. Sci. 2010;1187:148–171. doi: 10.1111/j.1749-6632.2009.05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol. Biochem. Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Schafer J, Armstrong TG, Dyer P. Cognitive functioning and length of abstinence in polysubstance dependent men. Arch. Clin. Neuropsychol. 2004;19:245–258. doi: 10.1016/S0887-6177(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch. Intern. Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol. Clin. Exp. Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Salas G, Schuff N, Norman D, Weiner MW, Fein G. Cortical metabolite in abstinent cocaine and cocaine/alcohol dependent subjects: an in-vivo proton magnetic resonance spectroscopic imaging study. Addict. Biol. 1999;4:405–419. doi: 10.1080/13556219971399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype? Alcohol. Clin. Exp. Res. 2008;32:1146–1158. doi: 10.1111/j.1530-0277.2008.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr. Top. Behav. Neurosci. 2011;2013:511–540. doi: 10.1007/7854_2011_131. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo T, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Parrish JK, Faulk MW, Arfken CL, Strahl-Bevacqua J, Manji HK. Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am. J. Psychiatry. 1999;156:1902–1908. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. NIAAA; Bethesda, MD: 2000. Neuropsychological vulnerabilites in chronic alcoholism; pp. 437–472. NIAAA Research Monograph No. 34. [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: role in the development of hypertension and end-stage renal disease. Curr. Opin. Nephrol. Hypertens. 2001;10:385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn. Reson. Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, Berger F, Grant I. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol. Clin. Exp. Res. 2001;25:924–934. [PubMed] [Google Scholar]
- Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug Alcohol Depend. 1998;50:39–45. doi: 10.1016/s0376-8716(98)00002-7. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am. J. Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith SS. Addictive Drug Survey Manual. NIDA Addiction Research Center; Baltimore, MD: 1991. [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: state of the art and future directions. Behav. Assess. 1990;12:77–90. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-Evaluation Questionaire. Consulting Psychologist Press; Palo Alto, CA: 1977. [Google Scholar]
- Stapleton JM, Morgan MJ, Phillips RL, Wong DF, Yung BC, Shaya EK, Dannals RF, Liu X, Grayson RL, London ED. Cerebral glucose utilization in polysubstance abuse. Neuropsychopharmacology. 1995;13:21–31. doi: 10.1016/0893-133X(94)00132-J. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Brain Vulnerability to Alcoholism: Evidence from Neuroimaging studies. NIAAA; Bethesda: 2000. [Google Scholar]
- Toennes SW, Thiel M, Walther M, Kauert GF. Studies on metabolic pathways of cocaine and its metabolites using microsome preparations from rat organs. Chem. Res. Toxicol. 2003;16:375–381. doi: 10.1021/tx025580n. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, Gonzalez-Saiz F, Fernandez-Calderon F, Bilbao-Acedos I, Perez-Garcia M. Self-regulation and treatment retention in cocaine dependent individuals: a longitudinal study. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lopez-Torrecillas F, Gimenez CO, Perez-Garcia M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychol. Rev. 2004;14:1–41. doi: 10.1023/b:nerv.0000026647.71528.83. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Vilar-Lopez R, Perez-Garcia M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug Alcohol Depend. 2007;86:139–146. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Berman SM, Hassid BD, Simon SL, Mandelkern MA, Brody AL, Monterosso J, Ling W, London ED. Differences in regional brain metabolism associated with marijuana abuse in methamphetamine abusers. Synapse. 2005;57:113–115. doi: 10.1002/syn.20155. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - III (WMS - III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]