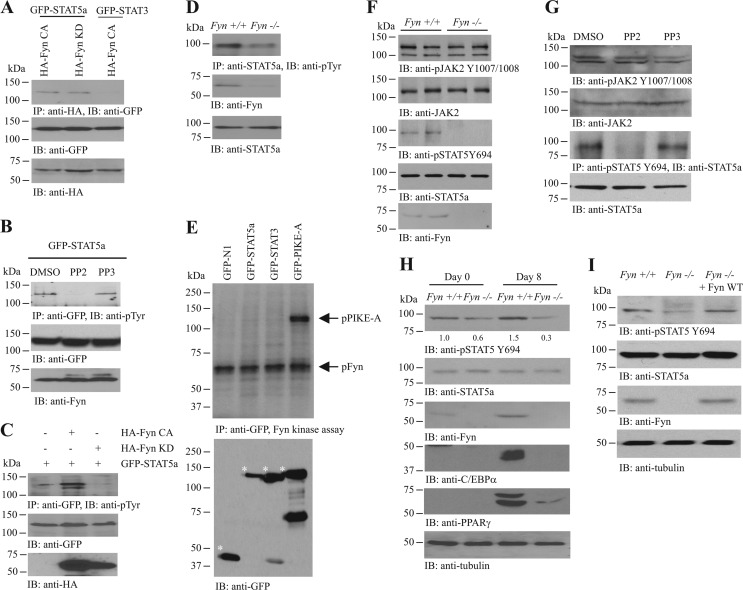
Fig 3.
Fyn enhances JAK2-mediated STAT5 phosphorylation. (A) Fyn interacts with STAT5a. Various GFP-tagged STAT constructs were cotransfected with HA-tagged Fyn mutants into HEK293 cells. Fyn proteins were immunoprecipitated, and the associated STAT proteins were analyzed by Western blotting (top panel). Expressions of GFP-STATs (middle panel) and HA-Fyn proteins (bottom panel) were also examined. (B) Inhibition of Fyn reduces STAT5a phosphorylation. GFP-STAT5a was transfected into differentiated 3T3-L1 cells, followed by DMSO, PP2 (5 μM), or PP3 (5 μM) treatment (24 h). The GFP-STAT5a proteins were then immunoprecipitated, and the total tyrosine phosphorylation was examined using antiphosphotyrosine (PY20) antibody (top panel). Expressions of the transfected GFP-STAT5a (middle panel) and endogenous Fyn (bottom panel) are also shown. (C) Overexpression of Fyn increases STAT5a total phosphorylation. Differentiated 3T3-L1 cells were transfected with a different combination of GFP-STAT5a, HA-Fyn CA, or HA-Fyn KD. GFP-STAT5a was then immunoprecipitated, and the total tyrosine phosphorylation of the protein was detected using PY20 antibody (top panel). Expressions of the transfected GFP-STAT5a (middle panel) and HA-Fyn (bottom panel) were also verified. (D) Total STAT5a phosphorylation is diminished in the inguinal WAT of Fyn−/− mice. STAT5a proteins in wild-type and Fyn−/− WAT were immunoprecipitated, and the total tyrosine phosphorylation of the protein was detected using PY20 antibody (top panel). Expressions of Fyn (middle panel) and STAT5a (bottom panel) were also verified. (E) Fyn does not phosphorylate STAT5a. Cell lysates from HEK293 cells overexpressing various GFP-tagged constructs were collected, and the GFP-tagged proteins were immunoprecipitated. The proteins were then incubated with recombinant Fyn kinase in the presence of 32P-γ-ATP and resolved in SDS-PAGE, and the protein phosphorylation was detected by autoradiography (upper panel). Expression of various GFP-tagged proteins (asterisked) was also examined (lower panel). (F) Reduced STAT5 Y694 phosphorylation in the inguinal WAT of Fyn−/− mice. Cell lysates were prepared from wild-type and Fyn−/− (3-month-old female) WAT, and the autophosphorylation of JAK2 (1st panel) and JAK2-mediated STAT5 phosphorylations (3rd panel) were examined. Expressions of JAK2 (2nd panel), STAT5a (4th panel), and Fyn (5th panel) were also examined. (G) Inhibition of SFK kinase activity diminishes JAK2-mediated STAT5a phosphorylation. Differentiated 3T3-L1 cells were treated with DMSO, PP2 (1 μM), or PP3 (1 μM) for 24 h. The STAT5a was then immunoprecipitated, and its phosphorylation by JAK2 was measured using anti-phospho-STAT5 Y694 antibody (3rd panel). Phosphorylation of JAK2 in the presence of SFK inhibitor was also examined (1st panel). Expressions of the endogenous JAK2 (2nd panel) and STAT5a (4th panel) were also examined. (H) STAT5a Y694 phosphorylation is reduced in differentiated Fyn−/− MEF. MDI inductions were performed in confluent MEF isolated from wild-type and Fyn−/− embryos (E13.5). Eight days after induction, cell lysates were collected and the STAT5a was immunoprecipitated. JAK2-induced phosphorylation of the precipitated STAT5a was examined using anti-phospho-STAT5 Y694 antibody (1st panel). Expressions of the endogenous STAT5a (2nd panel), Fyn (3rd panel), C/EBPα (5th panel), PPARγ (6th panel), and tubulin (7th panel) are also shown. The relative STAT5 Y694 phosphorylation values after normalization with the corresponding STAT5a levels are shown at the bottom of the 1st panel. (I) Enhanced STAT5 phosphorylation in Fyn-overexpressed Fyn−/− MEF. DNA plasmid (pRK5-Fyn) was delivered into Fyn−/− MEF by electroporation. Seventy-two hours after electroporation, the cells were stimulated with MDI cocktail. Eight days after induction, the cells were collected to examine STAT5 Y694 phosphorylation (1st panel). Expressions of STAT5a (2nd panel), Fyn (3rd panel), and tubulin (4th panel) were also determined.