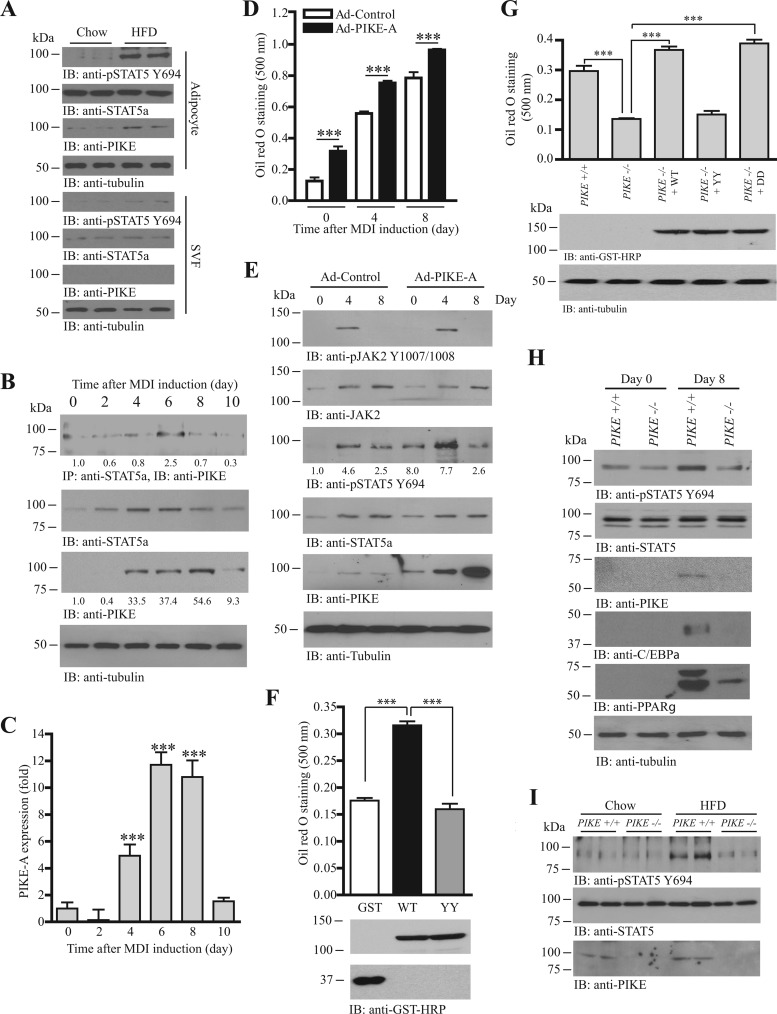
Fig 4.
PIKE-A enhances adipogenesis by promoting the JAK2-mediated STAT5a phosphorylation. (A) Increased PIKE-A expression and STAT5 phosphorylation in the adipocytes of HFD-fed mice. Immunoblots were performed using isolated adipocytes (adipocytes) and stromal vascular fractions (SVF) from chow- or HFD-fed mice. (B) PIKE-A/STAT5a association is upregulated during adipogenesis. Lysates of 3T3-L1 cells were prepared at different time points after MDI induction. Endogenous STAT5a was then immunoprecipitated, and the associated PIKE-A was examined (1st panel). Expressions of STAT5a (2nd panel), PIKE-A (3rd panel), and tubulin (4th panel) are also shown. The relative amount of coprecipitated PIKE-A at various time intervals after normalization with the expression level of STAT5a is indicated at the bottom of the 1st panel. Relative PIKE-A expression values after normalization with the corresponding tubulin levels are also shown at the bottom of the 4th panel. (C) PIKE-A expression is increased during adipogenesis. Total RNA was collected from 3T3-L1 cells at different time intervals after MDI induction. Real-time RT-PCR was then conducted to evaluate the expression of PIKE-A. Results are SEM of triplicate readings using cDNAs from three independent differentiation experiments and are expressed as fold inductions after normalization with the corresponding β-actin expression (***, P < 0.001; versus day 0; one-way ANOVA; n = 3). (D) Overexpression of PIKE-A in 3T3-L1 cells enhances adipogenesis. 3T3-L1 preadipocytes were infected with control adenovirus (Ad-Control) or adenovirus overexpressing PIKE-A (Ad-PIKE-A) for 48 h. The cells were then subjected to MDI induction. Lipid accumulation at different time intervals after MDI induction was quantified by oil red O staining (***, P < 0.001; Student's t test; n = 3). (E) PIKE-A overexpression enhances JAK2-mediated STAT5a phosphorylation in differentiating 3T3-L1 cells. 3T3-L1 preadipocytes were infected with control adenovirus (Ad-Control) or adenovirus overexpressing PIKE-A (Ad-PIKE-A) for 48 h. The cells were then subjected to MDI induction. Cell lysates were collected at different time points, and the phosphorylations of JAK2 on Y1007/1008 (1st panel) and STAT5a on Y694 (3rd panel) were examined. Expressions of JAK2 (2nd panel), STAT5a (4th panel), PIKE-A (5th panel), and tubulin (6th panel) are also shown. The relative values of STAT5 Y694 phosphorylation after normalization with the STAT5a expression are shown at the bottom of the 3rd panel. (F) Mutation of Fyn phosphorylation sites on PIKE-A abolishes its adipogenic activity. Control (GFP), mGST-PIKE-A WT, or mGST-PIKE-A YY plasmid was delivered into 3T3-L1 preadipocytes using electroporation. The cells were then subjected to MDI induction. After 8 days, lipid accumulation was evaluated by oil red O staining (top panel) (***, P < 0.001; one-way ANOVA; n = 3). Expressions of the transfected PIKE-A (middle panel) and GFP (bottom panel) were also verified. (G) Rescue of adipogenesis in PIKE−/− MEF. Various mGST-tagged PIKE-A plasmids [PIKE-A WT, PIKE-A Y682,774F (YY), and PIKE-A Y682,774D (DD)] were delivered into PIKE−/− MEF using electroporation. Seventy-two hours after the gene delivery, the cells were stimulated with MDI cocktail. Eight days after induction, the cells were fixed, stained with oil red O, and quantified (top panel) (***, P < 0.001; one-way ANOVA; n = 4). Expressions of the transfected plasmids (middle panel) and endogenous tubulin (bottom panel) were also determined. (H) JAK2-mediated STAT5a phosphorylation is reduced in differentiated PIKE−/− MEF. MDI inductions were performed in confluent MEF isolated from wild-type and PIKE−/− embryos (E13.5). Eight days after induction, cell lysates were collected and the JAK2-induced phosphorylation of STAT5 was examined using anti-phospho-STAT5 Y694 antibody (1st panel). Expressions of the endogenous STAT5a (2nd panel), PIKE-A (3rd panel), C/EBPα (4th panel), PPARγ (5th panel), and tubulin (6th panel) are also shown. (I) Reduced STAT5 Y694 phosphorylation in PIKE−/− WAT after HFD feeding. Cell lysates were prepared from the inguinal WAT of wild-type and PIKE−/− (6-month-old female) mice after chow or HFD feeding for 14 weeks, and the phospho-STAT5 Y649 (top panel) was examined. Expressions of the endogenous STAT5a (middle panel) and PIKE-A (bottom panel) are also examined.