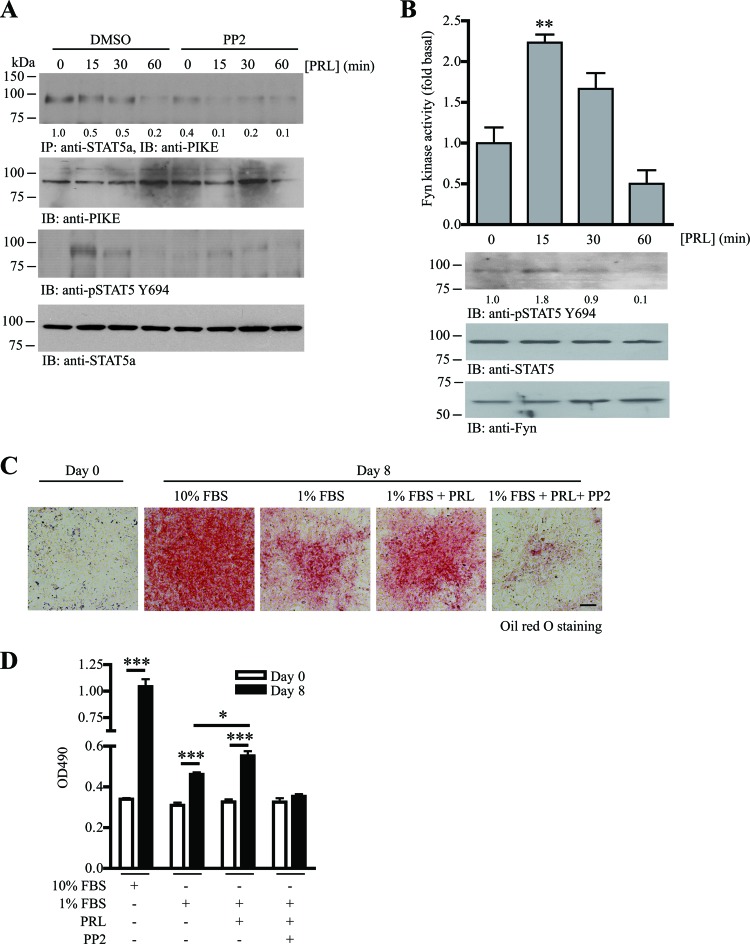
Fig 6.
PRL-induced PIKE-A/STAT5a tethering is mediated by Fyn activation. (A) PRL-stimulated STAT5a and PIKE-A phosphorylations are obstructed by SFK inhibitor. Fyn kinase activity in differentiated 3T3-L1 cells was inhibited by PP2 (1 μM) for 1 h. The cells were then stimulated with PRL (2 μg/ml) for various time intervals. The association of PIKE-A/STAT5a was examined using immunoprecipitation (1st panel). Phosphorylation on STAT5 Y649 was also examined (3rd panel). Expressions of the endogenous PIKE-A (2nd panel) and STAT5a (4th panel) are also shown. The relative amount of coprecipitated PIKE-A at various time intervals after normalization with the expression level of STAT5a is indicated at the bottom of the 1st panel. (B) PRL activates Fyn in adipocytes. Eight days after MDI induction, 3T3-L1 cells (serum starved for 24 h) were stimulated with PRL (2 μg/ml) for various time intervals. Cell lysates were then prepared, and Fyn was immunoprecipitated to measure its kinase activity on poly(Glu-Tyr) phosphorylation (1st panel) (**, P < 0.01; one-way ANOVA; n = 3). Phosphorylation of STAT5a on Y649 was detected as a positive control of PRL stimulation (2nd panel). Expressions of STAT5a (3rd panel) and Fyn (4th panel) are also shown. The relative values of STAT5 Y694 phosphorylation after normalization with the STAT5a expression are indicated at the bottom of the 2nd panel. (C) PRL-induced adipogenesis requires the activity of SFK. 3T3-L1 cells were subjected to MDI induction in the presence of 10% FBS, 1% FBS, 1% FBS with 1 μg/ml PRL, or 1% FBS with 1 μg/ml PRL and 1 μM PP2. Eight days after MDI induction, the cells were then fixed and stained with oil red O. Bar, 100 μm. (D) Quantification of the stained oil red O in the differentiated 3T3-L1 cells under various treatments shown in panel C (*, P < 0.05; ***, P < 0.001; versus day 0; Student's t test; n = 3).